Why manual transcription from eSource to EDC is now the industry’s biggest and most fixable bottleneck.
Clinical trials are entering a new era of technology innovation and adoption. We’ve seen AI-assisted site selection, decentralized models, real-time analytics, and fully digital workflows. Yet one of the most time-consuming, error-prone, and universally hated tasks in research has barely changed at all. Since the day eSource entered the industry, sites and sponsors have asked the same question: “Does the data automatically move into the EDC?”
For years, the answer was no. And the burden is bigger than many realize.
According to the RealTime Reports: eSource ROI Survey Summary (2025), 72% of sites still manually transcribe eSource data into EDC and 68% report discrepancies caused by manual transcription. This workflow slows decisions, inflates monitoring costs, and increases audit exposure.
That burden ends now with EDC Connect.
EDC Connect: The eSource to EDC Advantage
EDC Connect is RealTime’s breakthrough feature that enables sites to seamlessly map and export data from eSource into any Operational Data Model (ODM)-compatible sponsor EDC platform, significantly saving time and reducing errors.
This is the first time a site-centric solution has eliminated the duplicate entry burden using the same industry standard sponsors already use to configure their studies. It’s the simplest, cleanest path to eSource to EDC interoperability. Significantly, no additional manual re-entry or custom integration is required.
- Sponsor/CRO provides the ODM metadata file.
- Sites upload this metadata into RealTime-eSource.
- Visit data is automatically mapped.
- Sites export clean, structured data directly into the sponsor-required EDC.
The Impact of EDC Connect: Faster, Cleaner Data
Speed and accuracy define competitive advantages in clinical trials. Yet, interoperability has been the missing link in clinical research for decades. Eliminating duplicate data entry from eSource to EDC is one of the simplest and highest-impact improvements a site can make. With EDC Connect, RealTime delivers it, without added integrations.
When duplicate entry from eSource to EDC disappears, the benefits stack up quickly across time, quality, staffing, and compliance. Here’s what sites can expect from a fully connected eSource to EDC workflow:
1. Increased Staff Capacity Without Hiring
Even small inefficiencies add up across hundreds of visits. The 2025 RealTime Reports: eSource ROI Survey Summary shows that most sites spend over 10 minutes per visit on duplicate entry, and for many, it exceeds 11 minutes. A coordinator managing 10 patients weekly loses approximately 5+ hours every week to transcription alone. These are hours that should drive trial outcomes, not paperwork.
The data goes even further. Findings from the survey indicated that sites already save 6–20 minutes per visit with eSource. Removing transcription wins back even more time for essential trial activities.
2. Major Error Reduction
Transcription errors multiply downstream work. Even small inconsistencies ripple into operational risk. 68% of surveyed sites report transcription errors when re-entering data into EDC. Each transcription discrepancy from eSource to EDC creates additional queries, slows monitoring, and increases audit exposure.
By eliminating manual transcription, the source of most discrepancies, sites see fewer monitoring corrections and smoother audit cycles.
- Fewer transcription errors
- Fewer queries
- Faster lock-ready data
3. Measurable Operational ROI
The RealTime Reports: eSource ROI Survey (2025) highlights how significant the opportunity truly is:
- 88% of surveyed sites consider eliminating duplicate entry valuable
- 59% say very valuable
- Only 5% have achieved any type of automation
EDC Connect closes that interoperability gap from eSource to EDC for good.
Before vs. After: The EDC Connect Difference
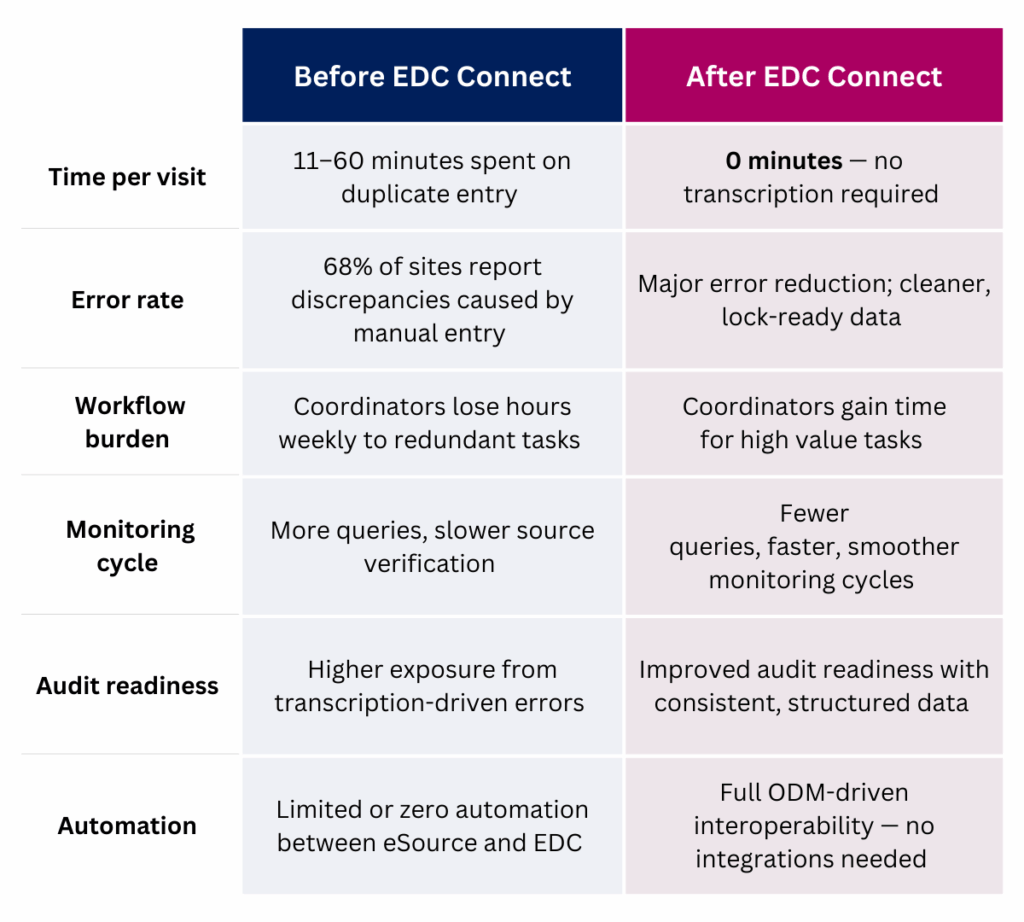
Final Thought: EDC Connect Delivers What eSource Promised
Altogether, every transcription touchpoint between eSource to EDC in clinical trials compounds risk, time, and cost. Research teams shouldn’t have to work twice as hard to produce data once.
Since the introduction of eSource, duplicate data entry has been the invisible tax slowing down clinical research. The 2025 RealTime Reports: eSource ROI Survey Summary made it clear: the burden is universal and costly. The good news is that today, it’s entirely avoidable. EDC Connect finally removes it. In turn, sites gain time, accuracy, and capacity. Sponsors gain cleaner data and faster insights. And patients benefit from trials that run more smoothly, confidently, and reliably.
Ready to see the platform in action? Book a demo or explore EDC Connect here.
Read More: Inside the 2025 eSource RealTime Report: Why Manual Data Entry Is Now the Industry’s Weakest Link
Read More: RealTime Reports: eSource ROI Survey Summary
EDC Connect — Frequently Asked Questions
Q: What is EDC Connect?
A: EDC Connect is RealTime’s new feature that allows sites to export clean, structured eSource data directly into any sponsor EDC system that supports the CDISC ODM standard, eliminating duplicate data entry from eSource to EDC.
Q: Does EDC Connect require custom integrations?
A: No. EDC Connect uses the same ODM metadata file sponsors already provide to configure their studies. No custom development or integration work is needed.
Q: Which EDC systems does EDC Connect work with?
A: Any EDC that is ODM-compatible, including the majority of modern sponsor systems. Sponsors/CROs supply the ODM metadata file as part of standard study start’up.
Q: How does EDC Connect improve data quality?
A: Removing manual transcription form eSource to EDC dramatically reduces:
- Transcription errors
- Queries
- Monitoring corrections
- Audit findings
This results in faster, cleaner, lock-ready data.
Q: What do sites need to do to use EDC Connect?
A: Just two things:
- Upload the ODM metadata file provided by the sponsor or CRO.
- Export mapped visit data directly into the sponsor’s required EDC.
Q: Does EDC Connect work for all visit types and studies?
A: Yes. EDC Connect maps study metadata and visit-level data from RealTime-eSource to the corresponding EDC structure—regardless of study size or phase.
Q: How does EDC Connect impact monitoring and audits?
A: By eliminating the most error-prone manual step, sites benefit from:
- Fewer queries
- Faster source verification
- Reduced monitoring cycles
- Greater audit confidence
Q: What makes EDC Connect different from other eSource to EDC approaches?
A: Here is what makes EDC Connect different:
- Uses industry-standard ODM
- No integrations
- No re-entry
- The first site-centric solution built on the same standard sponsors already use

