The adoption of eReg/eISF solutions in clinical trials can significantly improve outcomes across Academic Medical Centers (AMCs) and health networks, where efficient regulatory documentation management is essential. eReg/eISF solutions, electronic platforms specifically designed for regulatory document and process management in clinical trials, streamline the entire workflow, from document creation and secure storage to real-time access and electronic signatures. Fully compliant with key regulatory standards such as the FDA 21 CFR Part 11 and ICH GCP, these systems prioritize data security and integrity, ensuring that AMCs and health networks can confidently manage their regulatory responsibilities. The result? Reduced start-up timelines, better compliance, and improved trial management across the board. In this blog, we’ll explain how AMCs and health networks can accelerate clinical trial cycle times with eReg/eISF systems.
Common Challenges of Start-up Timelines in Clinical Trials
The start-up phase in clinical trials, from site initiation to patient recruitment, is often one of the most time-consuming stages. According to analysis in Spinger Nature, several factors contribute to extended start-up timelines, including the management of regulatory documents, contract negotiations, and Institutional Review Board (IRB) approvals. These steps often involve multiple stakeholders and require constant communication, which can introduce delays if managed manually or through disparate systems. In AMCs and health networks, these challenges are particularly pronounced due to the complexity and scale of the trials conducted. With multiple departments, investigators, and research coordinators involved, the management of essential regulatory documents becomes a logistical burden without the right-fit, purpose-built electronic solutions.
Furthermore, the study start-up phase for AMCs typically takes longer than community-based sites. Additional research has shown that the early stages of study initiation contribute significantly to lag time—the period from the discovery or start of research to its implementation in clinical practice. Findings indicate that variations in cycle time to the first patient occurs by site type, with AMCs and government-funded sites experiencing the longest delays, while physician practices tend to have the quickest timelines.1 Contributing to these additional delays is that AMCs have several layers of internal review, alongside institutional review boards (IRBs) or ethics committees and scientific review committees. Some larger AMCs in the U.S. also have operational review committees, which can delay site activation and impact study start-up. Of note, companies conducting oncology trials also typically report lengthy timelines as they rely on large AMCs to conduct their trials.
How Does eReg/eISF Reduce Clinical Trial Start-up Timelines?
By replacing paper-based workflows with electronic systems, eReg/eISF solutions eliminate many of the manual processes and communication bottlenecks that contribute to start-up delays. Here are five ways that eReg/eISF can accelerate clinical trial study start-up timelines:
Centralized Document Management
One of the key advantages of eReg/eISF is the centralization of all regulatory documents. In a traditional trial setting, regulatory binders are maintained physically or on fragmented systems, requiring manual tracking and handling. With eReg/eISF, all documents are stored in a single, secure platform, making it easy for stakeholders to access the necessary files anytime, anywhere.
Centralization speeds up the review and approval process, as investigators, sponsors, and CROs no longer have to wait for physical handovers or access to multiple platforms. This streamlined document access is especially valuable for large AMCs managing numerous trials and research teams. For example, on average, clients using RealTime-eReg/eISF reported that implementing the platform reduced the rate of study start-up by 20% contributing to faster start-up timelines across multiple trials.

Faster IRB Approvals and Contract Negotiations
Another time-consuming aspect of trial start-up is the process of obtaining Institutional Review Board (IRB) approvals and negotiating contracts with participating sites, both of which are critical but often lengthy steps. eReg/eISF platforms streamline and accelerate these processes by digitizing and automating many of the tasks involved.
For example, RealTime-eReg/eISF enables documents, such as contracts and IRB submissions, to be uploaded, tracked, reviewed, and signed electronically, providing all stakeholders with access to the latest versions of contracts and regulatory documents. This digital approach significantly reduces the back-and-forth and delays often associated with paper-based processes, allowing contracts to move from review to signature without the usual physical handovers. For AMCs, which typically manage large portfolios of trials, this functionality is invaluable as it not only shortens start-up timelines but also helps ensure consistent compliance with regulatory requirements.
Moreover, features such as automated notifications and approval workflows allow research teams to stay informed of pending signatures or IRB actions in real-time. This visibility into the status of each document helps prevent bottlenecks, ensuring that all parties remain aligned and reducing the potential for oversights that could delay trial initiation.
Improved Compliance and Audit Readiness
Regulatory compliance is non-negotiable in clinical trials and ensuring that all documents are audit-ready is a key priority during the start-up phase. eReg/eISF solutions strengthen compliance by establishing audit trails and providing real-time version control, which captures each change to a document along with time-stamped records of user activity. This transparency ensures that all actions are traceable, enabling research teams to meet FDA’s 21 CFR Part 11 and ICH GCP standards.
Altogether, eReg/eISF centralizes oversight of all regulatory documents, regardless of site location. This oversight ensures that AMCs are always prepared for audits and inspections, minimizing the risk of delays due to non-compliance. In fact, RealTime clients reported achieving five times the inspection readiness using eReg/eISF platforms.

Better Stakeholder Collaboration and Real-Time Access
The start-up phase often requires close collaboration between various stakeholders, including site staff, sponsors, and regulatory agencies. eReg/eISF platforms facilitate this collaboration by allowing real-time access to documents and providing tools for electronic signatures, version control, and audit trails. This real-time access ensures that there is no delay in sharing documents between stakeholders, accelerating the back-and-forth approval process for essential regulatory documents like informed consent forms, investigator agreements, and IRB submissions.
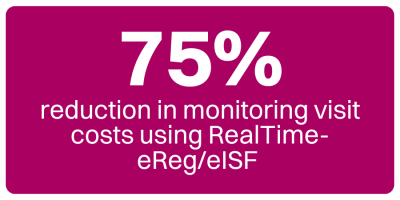
Incorporating eSignatures further speeds up the process, as documents can be signed electronically without needing physical presence. This feature was especially useful during the COVID-19 pandemic when many stakeholders worked remotely and travel restrictions limited in-person interactions. During the pandemic, eReg/eISF enabled business continuity, allowing clinical trials to continue without disruption by enabling remote access to critical documents. In fact, clients who have already adopted RealTime-eReg/eISF have reported a 75% reduction in monitoring visits costs.
Integrations with Other Clinical Trial Management Systems
eReg/eISF systems don’t operate in isolation and can integrate with other critical platforms. For instance, an integration with a Clinical Trial Management Systems (CTMS) enables seamless data sharing between systems that reduces many of the redundancies and errors that occur during manual data transfers. For AMCs and health networks, where multiple trials may be running simultaneously, integration allows for better coordination between site staff and trial sponsors, ensuring that all parties are aligned on timelines, tasks, and regulatory requirements. This coordination plays a crucial role in reducing the time needed to progress from site initiation to patient recruitment.
For example, integrating eReg/eISF with CTMS maintains compliance and audit readiness by reducing the need for manual data entry and document retrieval, which lowers compliance risks. This integration also accelerates study start-up by making essential documents, such as training certificates, regulatory approvals, and investigator credentials—immediately accessible for each new trial. Furthermore, it enables smooth data exchange between platforms, which minimizes errors and ensures regulatory requirements are consistently met.
Conclusion
The adoption of eReg/eISF solutions systems can reduce trial timelines in clinical trial particularly in AMCs and health networks, where complexity and scale often lead to delays. By centralizing document management, improving collaboration, integrating with other clinical trial systems, and strengthening compliance, eReg/eISF significantly reduce the time it takes to move from site initiation to patient recruitment.
FAQs
1. What are the initial implementation costs and time commitment required to adopt eReg/eISF platforms?
Implementation costs for eReg/eISF solutions can vary based on the scope and size of the institution. Generally, AMCs and health networks should consider budgeting for data migration, training, and any necessary integration with existing systems. Initial setup often takes a few weeks, depending on the complexity of existing workflows and data migration needs. RealTime works closely with partner-clients to streamline the transition and offer tailored support to minimize disruption.
2. How does eReg/eISF adoption impact staff workload and user experience in AMCs?
RealTime-eReg/eISF is purpose-built to reduce manual administrative tasks, thus lightening staff workload over time. While there may be a brief learning curve, the systems generally enhance user experience by centralizing document access, enabling real-time updates, and automating routine tasks. Feedback from other AMCs and large site networks highlight improved efficiency and less time spent on compliance-related tasks, allowing staff to focus on core responsibilities.
3. What specific challenges might AMCs face during the transition to eReg/eISF systems?
Some AMCs may encounter initial challenges such as training staff on the new system, ensuring interoperability with other clinical trial management platforms, and adjusting to changes in workflows. To address this, RealTime offers comprehensive onboarding support, and many systems are designed to integrate smoothly with existing platforms like CTMS to ease the transition. Planning for phased implementation and having designated support teams in place can help mitigate these challenges.
4. How does eReg/eISF directly benefit patient recruitment beyond start-up timeline improvements?
Centralized and accessible documentation helps maintain efficient communication with recruitment staff, ensuring they are fully equipped to engage participants without administrative delays, which improves patient recruitment timelines.
5. What are the security measures in place to protect sensitive data on eReg/eISF platforms?
eReg/eISF platforms are designed with rigorous data security measures to protect sensitive trial data. Most platforms are fully compliant with FDA’s 21 CFR Part 11 and ICH GCP, with built-in security features like user access control, data encryption, audit trails, and regular data backups. This ensures that only authorized users can access documents, and all interactions are logged to maintain data integrity and protect against unauthorized access.
About RealTime eReg/eISF
From the pioneers of eDocs and Complion eISF, our user-friendly electronic document management and regulatory systems are built for clinical research sites and academic medical centers. Eliminate paper clutter and simplify document storage, retrieval, and collaboration across site teams or departments with RealTime-eReg/eISF.
Read More: eReg/eISF in Clinical Trials – 8 Trends Driving Adoption
Read More: Leading Clinical Trials with Precision: Tracking Performance that Delivers Results
1 Lamberti MJ, Brothers C, Manak D, Getz K. Benchmarking the study initiation process. Ther Innov Regul Sci. 2013;47:101–109.

