
Advanced data collection and management.
Site-friendly eSource for fewer errors.
Remote trial management and monitoring.
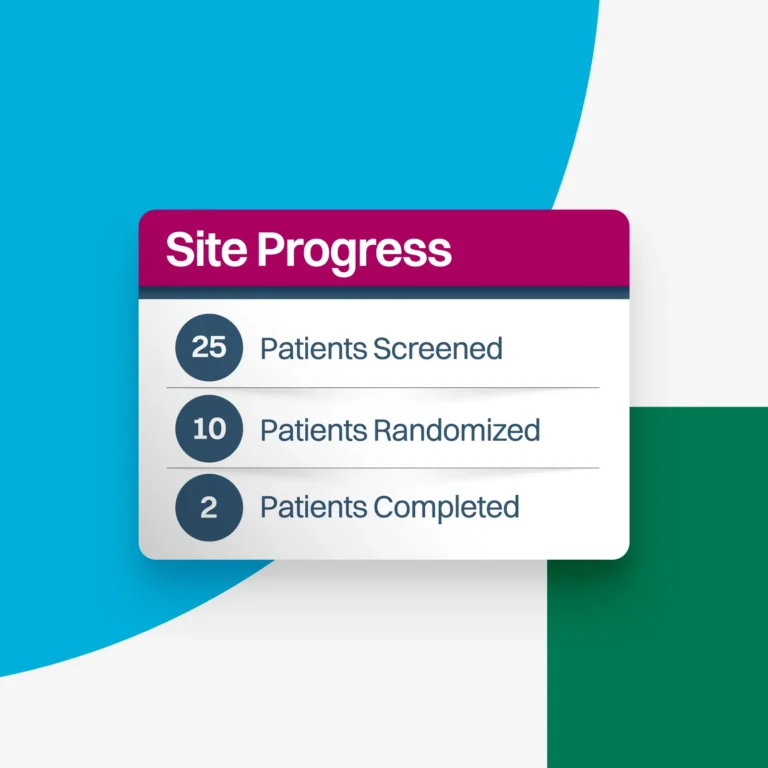
Real-time reporting to analyze and review site progress.
Manage your entire clinical trial from a single platform and eliminate the need for legacy EDC systems.
Leverage RealTime-eSource to reduce transcription delays and accelerate data reporting for faster study completion and quicker access to critical research findings.
Minimize human error and ensure data integrity with built-in data validation checks. RealTime helps you maintain the highest quality data standards for reliable results.
Robust audit trails allow users to reconstruct study events and organize the auditing process.

Leverage a user-friendly eSource system already adopted by thousands of global research sites.
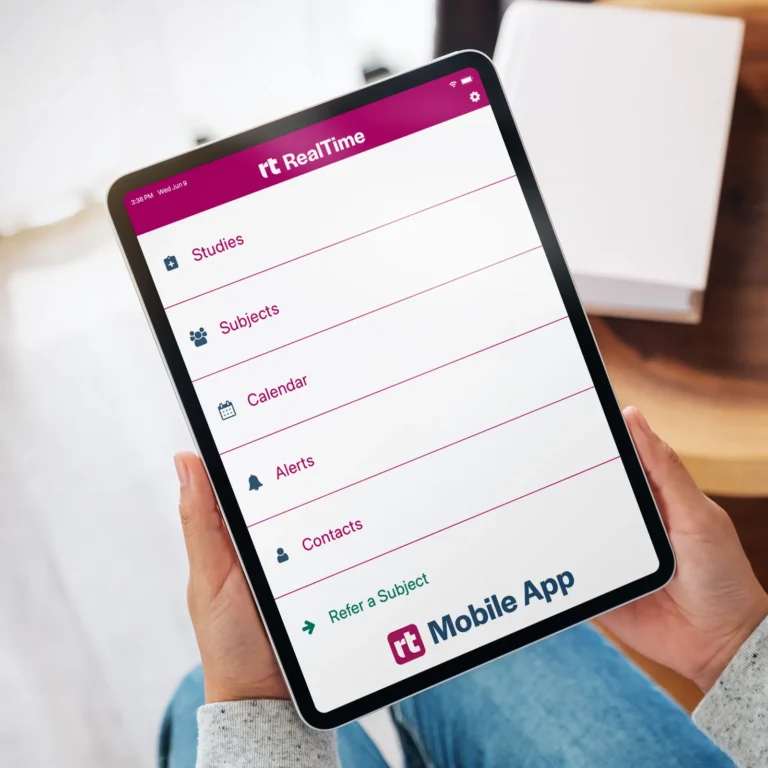
Empower users to collect data on-the-go with our site-specific mobile app.
Tailor visit workflows to seamlessly match individual site procedures.
Improve investigator and sponsor oversight for better outcomes.
Dramatically reduce travel expenses for monitors and CRAs.
Gain immediate visibility into site progress and data as visits occur.
Built-in query system to review, track and, query data points.
Maintain greater visibility for safer and more effective clinical trials.
Customize a DecenTrial program that perfectly fits your specific research needs.
Modernize data collection and roll-up with advanced eSource tools.
Experience the power of fully remote trial oversight through advanced dashboards.
RealTime eClinical Solutions is a leading, enterprise-grade technology provider facilitating global clinical trials with a comprehensive eClinical platform. Purpose-built for clinical research sites, site networks, academic medical centers, sponsors, and CROs, the platform goes beyond traditional CTMS to empower research and business workflows for modern-day clinical trials.
| Cookie | Duration | Description |
|---|---|---|
| cookielawinfo-checbox-analytics | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Analytics". |
| cookielawinfo-checbox-functional | 11 months | The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". |
| cookielawinfo-checbox-others | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Other. |
| cookielawinfo-checkbox-necessary | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookies is used to store the user consent for the cookies in the category "Necessary". |
| cookielawinfo-checkbox-performance | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Performance". |
| viewed_cookie_policy | 11 months | The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. It does not store any personal data. |