Academic Medical Centers (AMCs), sites, and health networks are constantly striving to streamline processes, reduce timelines, and improve outcomes. Central to achieving these goals is the ability to manage trials effectively, ensuring all key players have the data they need to make informed decisions. If you are looking to view, manage, and optimize site performance from beginning to end, and break down silos that can often hinder communication and slow trial progression, it’s time to get familiar with RealTime-Devana. From study start-up to execution, we’ll unpack how organizations conducting clinical trials can centralize visibility and performance transparency for better trial outcomes.
The Risk of Operating in Silos
Data Silos
Lacking centralized visibility and performance transparency in clinical trials can lead to several significant issues. One of the primary concerns is the presence of data silos. Without a unified view, data remains fragmented across various systems, making it difficult to access comprehensive information and insights. This fragmentation can hinder decision-making and limit the ability to respond quickly to changes or emerging issues.
Poor Communication and Collaboration
Inefficient communication is another critical problem. When systems are disconnected, it can result in miscommunication among team members and stakeholders. This miscommunication often leads to duplicated efforts, misunderstandings, and delays in project timelines, further complicating the trial process.
Increased Risk of Errors
Additionally, there is an increased risk of errors associated with relying on multiple platforms and manual data entry. These inaccuracies can significantly impact trial outcomes and result in costly delays or regulatory complications. Furthermore, without centralized performance metrics, teams may struggle to monitor progress in real-time, preventing early identification of potential problems and resulting in a reactive rather than proactive management approach.
Inefficient Resource Allocation
Resource allocation issues are another consequence of limited visibility. This constraint can hinder effective resource allocation, making it challenging to optimize team performance and ensure that critical tasks are prioritized appropriately.
Finally, all these factors can contribute to longer trial durations, ultimately delaying the time it takes to bring new treatments and therapies to market. Alongside hybrid/decentralized trials and complex studies, keeping trials on track demands a unified approach to data management and performance oversight. To address these challenges, a centralized system that offers real-time visibility and transparency across all phases of a trial is essential. That’s why nine of the ten industry’s leading site networks have already adopted RealTime-Devana for business intelligence, study start-up, and project and pipeline management.

The Solution: RealTime-Devana
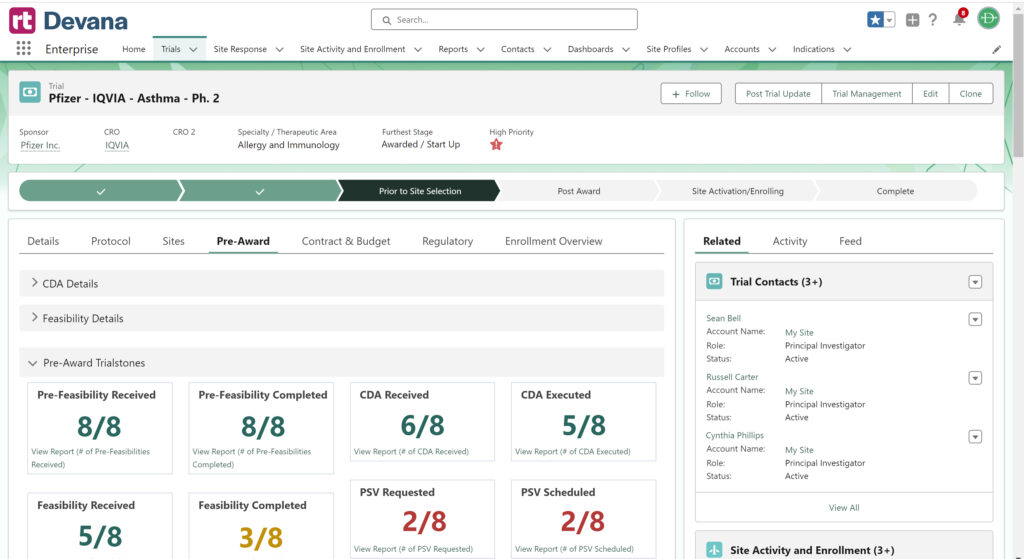
RealTime-Devana is a data analytics platform that improves clinical research site management through the capture and display of key timing and performance metrics, from study start-up to execution. Here’s how Devana breaks down silos, establishes centralized visibility, and drives data-driven decision-making for faster, more successful results.
Study start-up can be one of the most time-consuming aspects of a clinical trial. It involves numerous stakeholders and tasks, including site selection, regulatory submissions, budget negotiations, and contract execution. This phase can also lead to delays that negatively impact the study timeline. If you’re overseeing clinical trials, then you’ll value simplifying the entire start-up process and consolidating all relevant information in one location. Centralizing visibility and oversight make it easier for clinical trial decision-makers to track progress and identify bottlenecks. With Devana, study teams benefit from increased visibility and accountability throughout the study start-up process, including enrollment performance and overall trial progress.
Devana streamlines, centralizes, and automates study start-up in clinical trials. Study teams can quickly respond to notifications about new trial opportunities and receive timely task alerts related to specific studies. Other key features include threaded chat that simplifies communication, unlocking real-time collaboration and seamless document sharing no matter where your teams are located. Plus, to help clinical trial decision-makers with end-to-end oversight, Devana also integrates with the industry’s leading Clinical Trial Management Systems (CTMS), as well as other mission critical systems to create a seamless flow of data that can be accessed in real time.
Visibility in Site Selection and Feasibility
Selecting the right sites is critical to the success of any clinical trial. Sponsors and CROs need to ensure that the selected sites have the capability, capacity, and patient access to conduct the trial successfully. RealTime-Devana simplifies this assessment process by providing comprehensive performance profiles on sites such as PI experience, therapeutic areas, capabilities, and more.
Capacity Monitoring
Devana provides real-time visibility into a site’s current capacity and workload by continuously tracking study-specific metrics, including the number of ongoing trials, active enrollment figures, and resource allocation. This level of transparency allows study teams to monitor how much work is being handled at any given time and how close the site is to reaching its capacity. With this real-time data, Devana helps ensure that sites are not overloaded. This allows teams to make data-driven decisions about whether they can take on new studies without compromising the performance of existing ones.
“We talked with some different sites as we were trying to determine whether or not to try Devana. We were sold on the fact that one site showed us the reports they run for staffing. They look at where they’re bringing up studies and determine their staffing levels based on that and what those reports tell them.” – RealTime-Devana Partner-client
Performance History
The system tracks key metrics such as enrollment rates, study timelines, and quality outcomes, giving sites a complete view of their past trial performance. Sponsors use these insights to assess which sites are best suited for future studies, selecting top-performing sites based on real data. By offering transparency into these critical metrics, Devana helps sites highlight their strengths, making it easier for sponsors to make informed decisions about site selection for upcoming trials.
Clinical Trial Execution: Real-Time Monitoring and Adaptive Management
Once a trial moves beyond start-up, the focus shifts to execution, where the ability to monitor and adjust in real time is critical. Devana ensures that every stakeholder has access to up-to-date information, allowing clinical trial decision-makers to make informed decisions and adjustments throughout the trial.
Real-Time Data Access
Research teams or departments using Devana can access real-time data on study progress, enrollment metrics, and site performance. This provides greater visibility and control throughout the clinical trial process. Using Devana’s automated tools, organizations can also significantly reduce dependence and time spent on labor intensive spreadsheets or using other disconnected tools. On average, this streamlined approach leads to a 30% of time savings per week.

Streamlined Reporting and Analytics
Devana’s reporting and analytics capabilities allow clinical trial decision-makers to quickly generate reports and track study progress. Common reports include:
- Site performance reports, which analyze metrics specific to each site, such as enrollment rates, patient demographics, and milestone achievements, enabling effective evaluation of site performance.
- Trial progress reports provide an overview of trial status, detailing enrollment progress, milestone completion, and any outstanding tasks or issues that need addressing.
- Operational metrics reports, which monitor operational efficiencies by tracking turnaround times for site responses, approval durations, and other key performance indicators (KPIs).
The result? Informed data-driven decision-making and improved trial outcomes.
Real-World Proof: RealTime-Devana in Action
In one Devana case study, the Medical University of South Carolina (MUSC) was challenged with evaluating hundreds of study opportunities for feasibility. This process required assessing whether each trial was a good fit based on physician investigator expertise and staff availability. However, the Feasibility Analysis Team lacked visibility into both historic and currently running trials, making it difficult to gauge past successes and current investigator capacity. Furthermore, the study selection and start-up processes were tied to outdated spreadsheets, which slowed operations and hindered their ability to track and provide essential KPIs.
With Devana, MUSC gained the ability to capture and analyze both historic and real-time trial performance metrics. This led to significant improvements in the feasibility analysis and study selection processes. The platform provided a unified and automated solution for managing study workflows and milestones, allowing the research team to streamline operations across the board.
In just a few weeks, data from over 1,400 clinical trials was organized and imported into the system. The entire research staff was unified on a common data analytics platform, offering transparency into both historical and current trial data. As a result, the volume of trial opportunities tracked in the pipeline doubled, and detailed metrics on over 250 active trials were available. With Devana, MUSC could now track key metrics in real time, including enrollment numbers, contract met percentages, study stages, and milestone completions.
Why Devana? Performance Transparency and Data-Driven Decision Making Across All Phases
The ability to make data-driven decisions can mean the difference between a study that stays on track and one that faces costly delays. With Devana, AMCs, sites, and health networks can track enrollment, activity, timing, and performance based on trial, study team, sponsor, and more. Ultimately, the platform allows for informed decision-making at every stage of the trial. In addition to these high-level benefits, Devana offers several specific tools and features that empower research teams to make the most of their data:
Data Analytics: With built-in analytics capabilities, Devana helps research teams turn data into actionable insights. By analyzing site performance, patient recruitment trends, and other key data points, teams can optimize study execution and address challenges before they escalate.
Comprehensive Dashboards: View customizable dashboards that display key metrics such as enrollment progress, site performance, and regulatory document completion. These dashboards provide at-a-glance insights that help teams stay on top of study milestones and identify areas for improvement.
Benchmarking: Devana allows for performance benchmarking, enabling teams to compare their study’s progress against previous trials. This ensures that trials are on track and provides an opportunity to implement best practices from high-performing studies.
Centralized Coordination: When selecting a site for a clinical trial, Devana allows central teams to search its entire network, filter by essential capabilities, and directly send opportunities. This centralized system eliminates the need for emails or physical documents, and it tracks responses and turnaround time metrics. Once a trial is underway, teams can easily share protocols, contracts, and other essential trial documentation, progress and milestones with one another, regardless of their location. This transparency helps teams align clinical trial activities across multiple therapeutic areas and ensure better utilization of resources.
Final Thoughts
The data is clear – Devana has helped organizations to centralize visibility and performance transparency, increasing ROI by 60% and reducing contract and budget turnaround time by 76% on average.
Devana serves as the backbone of the clinical trial tech stack, offering integrations that unify siloed clinical trial data – from IRB systems, contract and budget tools, regulatory systems, and more – into a single, centralized system. By connecting these previously siloed systems in an easy-to-use platform, teams can centralize visibility and accelerate trial cycle times.
About RealTime-Devana
RealTime-Devana is a holistic business intelligence platform designed specifically for research site organizations, driving transparency and accountability across all functional groups. From pipeline management and streamlined study start-up through to historical trial metrics, Devana provides one source of truth for all your clinical trial data and processes. Improve turnaround times and stand out to CROs and sponsors with Devana’s powerful workflow improvements and data analytics.

