RealTime Reports: Closing the Gap Between eSource Data Capture and Data Flow.
Modern sites are under relentless pressure: more trials, tighter timelines, higher expectations for data integrity, and audit readiness. Traditional paper-based or hybrid workflows simply can’t keep up. Electronic source data capture (eSource) is now rapidly becoming the gold standard for high-performing sites.
In the latest RealTime Reports: eSource ROI Survey Summary (2025), which captured insights from 255 clinical-research professionals globally, RealTime unpacked the real ROI of eSource, from streamlined workflows to delivering measurable operational and compliance gains.
Data Quality: The Hidden Cost of Paper vs eSource
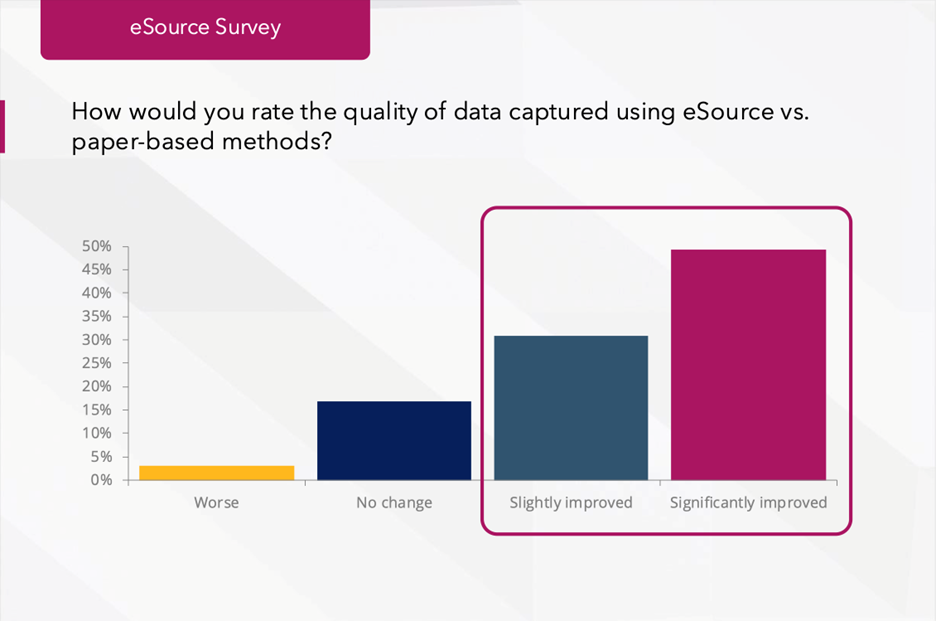
While paper-based workflows may feel familiar, they remain one of the weakest links in data accuracy. Every handwritten note or retyped entry introduces a potential risk of missing fields, illegible text, inconsistent formatting, or delayed documentation. In contrast, eSource captures data once, at the point of care, standardizing documentation and automatically time-stamping every entry. This eliminates many of the risks inherent to paper or hybrid workflows and ensures that data is consistent, complete, and verifiable.
According to the RealTime Reports survey, 83% of sites reported improved data quality after implementing eSource. Sites also noted fewer monitoring queries and less time spent reconciling discrepancies between systems.
Why this matters: Data quality is the foundation of every successful trial. Cleaner data means fewer downstream queries, faster source data verification, and less time spent reconciling discrepancies between systems. For sponsors and CROs, that translates to higher trust and reduced monitoring costs. Sites that continue relying on manual methods face mounting inefficiencies and the perception of being behind the curve in operational maturity.
Real Time + Real Minutes Saved
Capturing source data electronically goes beyond convenience. These time savings ripple outward, freeing staff to focus on participant care, data accuracy, and sponsor collaboration. Sites using eSource also report measurable improvements in protocol adherence, audit readiness, and monitoring efficiency.
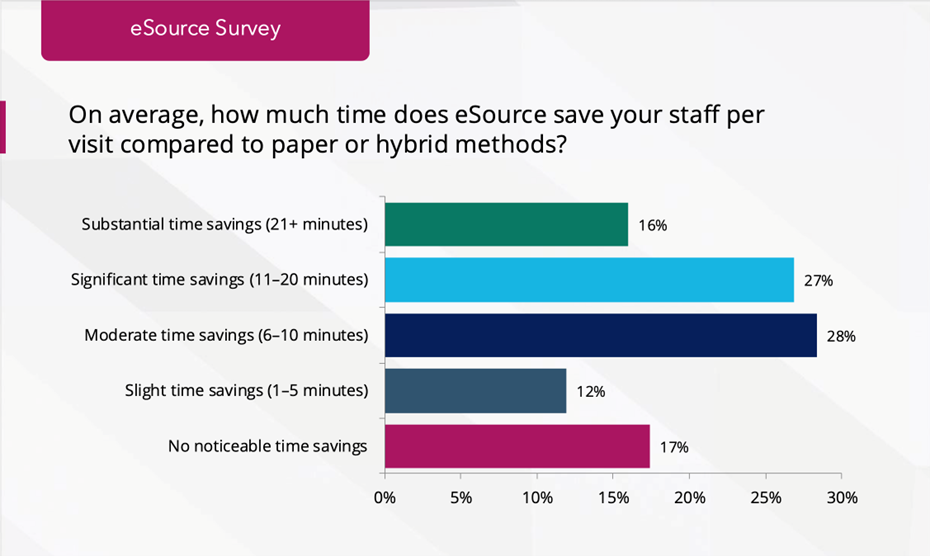
71% of sites report saving 6+ minutes per visit when using eSource.
- 28% saved 6–10 minutes
- 27% saved 11–20 minutes
- 16% saved more than 20 minutes
Why this matters: Even 15 minutes saved across 10 patients equals 2.5 hours of coordinator capacity. Scaled across multiple trials, that’s days, or even weeks of staff time reclaimed annually. Those saved hours can be redeployed to recruitment, retention, quality review or value-added tasks, not transcription or clean-up.
Compliance & Audit Readiness
Beyond speed, eSource strengthens compliance and audit posture, both of which are critical differentiators in today’s regulatory environment.
- 65% of audited sites post-eSource reported no or only minor audit findings.
- 59% attribute that success at least in part to eSource implementation.
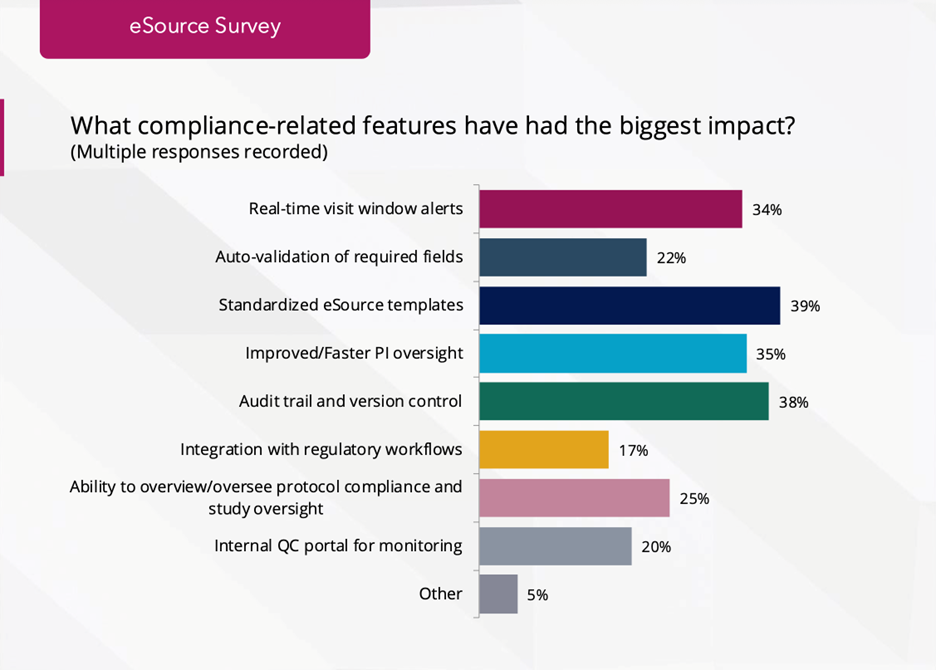
- Compliance-enablement features cited:
- Standardized templates: 39%
- Audit trail & version control: 38%
- Real-time PI oversight: 35%
- Visit-window alerts: 34%
Why this matters: Regulatory scrutiny is intensifying, and sponsors are prioritizing sites that can demonstrate remote monitoring capability and full traceability with audit trails. But reducing risk is only part of the story. These capabilities also signal operational maturity and reliability, making sites far more attractive to sponsors and CROs. In competitive site selection, a strong compliance infrastructure can tip the scales in your favor.
The Remaining Weak Spot: Data Duplication & Manual Transcription
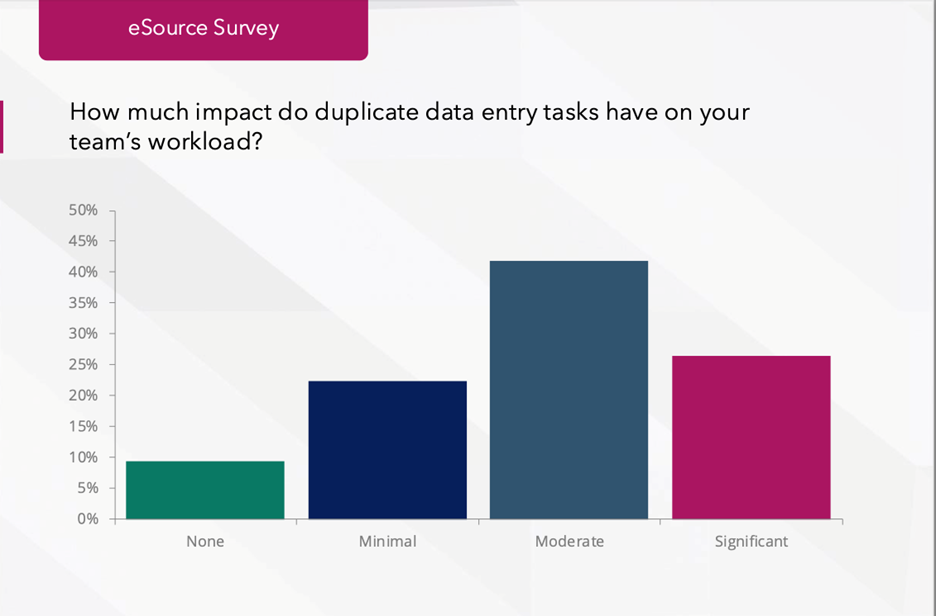
Even as eSource becomes the standard, manual data transcription remains a quiet drag on site performance. According to the RealTime Reports: eSource ROI Survey, 68% of sites report duplicate entry still impacts their workload, and one in four call the impact significant.
Furthermore, over 60% of sites still spend more than 10 minutes per visit reconciling or re-entering data from eSource to EDC. That’s time that could otherwise support patient care, recruitment, or quality oversight. Another 72% of surveyed sites continue to transfer data from eSource to EDC manually. Across hundreds of visits per study, that burden translates into hours of lost coordinator time per week, lower morale, and rising turnover.
Importantly, manual re-entry increases exposure to transcription errors and protocol deviations, two of the most common root causes of audit findings across regulated trials. When every duplicate entry adds risk, the cost of inefficiency is measured not just in time, but in compliance.
“Every extra layer of re-entry compounds stress on already stretched teams,” concludes Founder and Chief Strategy Officer at RealTime, Rick Greenfield. “The simplest way to relieve that burden isn’t to ask coordinators to work harder. It’s to remove the task altogether.”
Why this matters: Manual re-entry between systems keeps sites trapped in legacy workflows that slow innovation and cloud visibility. As long as eSource data requires human re-entry into downstream systems, sites are only realizing half the value of digital transformation. The next phase of progress will be about intelligent data flow. The real frontier of eSource lies in integration, turning isolated systems into a unified, automated research network.
Wrap Up
The RealTime Reports: eSource ROI Survey Summary (2025) reinforces what high-performing sites already know: eSource delivers measurable operational, compliance, and financial value. And for those who choose to lead, the payoff is significant. But the work isn’t finished.
The next chapter in eSource will focus on closing this last gap, automating the connection between data capture and data flow to finally unlock the full promise of the platform. As integration technology matures, the path forward is a direct, validated data flow between eSource and EDC. For sites, that means fewer errors, faster monitoring, and more time for what matters most.
Access all key findings, charts, and expert insights. Download the full report right here: RealTime Reports: eSource ROI Survey Summary 2025
Read More: RealTime Reports: Negotiating Sponsor Reimbursement for Site-based Technology
Read More: RealTime-eSource: Fueling 28x Revenue Growth at the University of the Sunshine Coast
FAQ: The State of eSource in 2025
Q: What is eSource in clinical trials?
A: eSource, or electronic source data capture, replaces traditional paper-based documentation by allowing study data to be entered directly into a digital system at the point of care. This approach eliminates transcription errors, reduces redundant data entry, and improves real-time oversight across studies.
Q: What did the RealTime Reports: eSource ROI Survey Summary reveal?
A: The 2025 survey, which gathered responses from 255 clinical research professionals worldwide, confirmed that eSource adoption is delivering measurable benefits in efficiency, compliance, and data quality. The report highlights that eSource is now the new operational baseline for high-performing research sites.
- 83% of sites reported improved data quality.
- 71% saved 6+ minutes per visit.
- 65% of audited sites reported no or only minor findings post-eSource implementation.
Q: Why are paper-based workflows still a challenge?
A: Paper-based or hybrid workflows continue to introduce errors such as missing entries, illegible notes, and inconsistent formatting. These inefficiencies not only slow operations but also increase compliance risk. According to the RealTime survey, sites using eSource consistently report cleaner data and fewer monitoring queries compared to those using manual methods.
Q: If eSource is so effective, what challenges remain?
A: Even as eSource adoption expands, manual data transcription between systems remains a key obstacle. The RealTime survey found that 68% of sites still experience workload impact from duplicate data entry, and over 60% spend more than 10 minutes per visit reconciling or re-entering data from eSource to EDC. This highlights the industry’s next focus area—creating seamless, automated data flow across systems.
Q: How does eSource improve audit readiness and compliance?
A: eSource creates a complete, time-stamped digital record of every entry, enabling full traceability and real-time oversight by investigators and monitors.
The RealTime Reports survey found that 59% of sites attributed improved audit outcomes directly to eSource adoption. Standardized templates, audit trails, and visit-window alerts all contribute to stronger compliance posture and reduced findings.
Q: What’s next for eSource technology?
A: The next chapter of eSource is about integration. As validated data standards and automation tools mature, sites will soon be able to move data directly between eSource and EDC systems without manual re-entry. This evolution will eliminate duplicate work, reduce risk, and create a unified ecosystem that benefits sites, sponsors, and CROs alike.
Q: Where can I access the full report?
A: You can download the full RealTime Reports: eSource ROI Survey Summary (2025) under the Resources section of the RealTime website to explore all key findings, charts, and expert insights that define the new standard for operational excellence in clinical research.

