In clinical research, budgets are always under pressure. So, when a sponsor, CRO, or vendor offers a “free” system, whether it’s a Clinical Trial Management System (CTMS) or an eRegulatory (eReg) platform (also called an electronic Investigator Site File, or eISF), it can sound like a welcome relief.
But as many Academic Medical Centers (AMCs), site networks, and research sites have discovered, “free” often comes with a price tag measured not in dollars upfront, but in lost time, hidden inefficiencies, data exposure, and increased compliance risk. The truth is simple: what looks like savings today can become a liability tomorrow. Let’s unpack the hidden cost of “free” eClinical platforms in clinical trials.
5 Hidden Costs of “Free” eReg/eISF Platforms
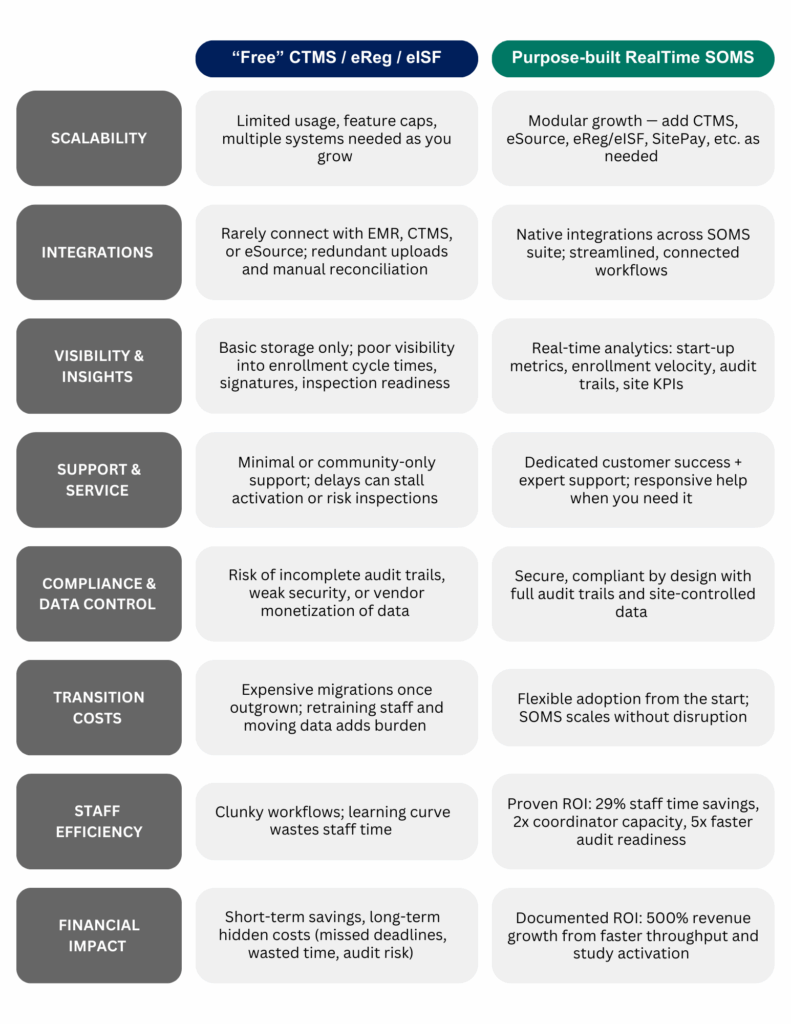
1. Limited Scalability
Most free systems cap usage or restrict functionality. That might work for a handful of studies, but as trial volume grows, sites quickly hit a ceiling. Expanding often means either paying for an upgrade or splitting operations across multiple systems, both of which add cost and complexity.
2. Integration Gaps
Free tools rarely connect seamlessly with CTMS, EMR, or eSource platforms. Without integrations, staff end up uploading the same documents multiple times, reconciling versions manually, and managing redundant workflows. These workarounds drain time and increase the risk of error.
3. Visibility Limits
While free platforms often cover the basics of document storage and compliance, they usually stop short of delivering the deeper analytics that sites need for complete oversight. Metrics like enrollment cycle times, signature turnaround, and inspection readiness aren’t easily surfaced, leaving sites struggling to address bottlenecks or performance.
4. Support & Service Gaps
When a system is free, premium support isn’t part of the package. Instead, users are left with self-service portals, community forums, or long support queues. In regulated research, a delayed response can mean delays in trial activation or added inspection risk.
5. Costly Transitions
What starts as “free” often leads to expensive migrations later. Once a site outgrows the free tier, moving documents, retraining staff, and aligning processes with a new system requires significant time and resources.
The Hidden Costs of “Free” CTMS Offers
The same dynamic applies to free CTMS platforms:
- Functionality trade-offs: stripped-down versions lack financial tracking, robust reporting, or remote monitoring support.
- Vendor lock-in: once your data is inside, migrating is painful and expensive.
- Reporting blind spots: free CTMS tools don’t deliver the level of enrollment, financial, or operational insights required for enterprise-grade decision-making.
- Data monetization risks: in some models, your operational data is the currency that funds the “free” system.
Why Modularity Matters
The alternative isn’t overspending on a bloated system. With RealTime’s Site Operations Management System (SOMS), sites, networks, and AMCs can start with the functionality they need most and add additional eClinical solutions as they grow. That flexibility means:
- Quick wins without overwhelming staff capacity
- Department- or site-specific rollouts
- A foundation that scales across studies and phases
It’s not about doing everything at once. It’s about building smart, connected workflows that give sites control, compliance, and confidence.
The Real ROI of Purpose-Built Systems
Case studies from leading networks show the measurable value of unified systems:
- 29% weekly staff time savings
- 500% revenue growth through faster throughput and study activation
- 2x coordinator capacity
- 5x faster inspection readiness with built-in audit trails
These aren’t theoretical benefits — they’re the real costs avoided when sites invest in purpose-built systems from the start.
Questions to Ask Before Accepting “Free”
If you’re offered a free eReg/eISF or CTMS, ask:
- Who owns and controls our data?
- How are compliance and audit trails guaranteed?
- What integrations exist (or don’t)?
- What’s the cost of retraining staff when it doesn’t fit our workflows?
- How will we scale when trial volume increases?
If the answers aren’t clear, the risk is.
Final Takeaway: Free Isn’t Free
Free CTMS or eReg/eISF systems may look attractive upfront, but the hidden costs, like lost time, poor integration, audit exposure, and loss of data control, are far higher.
The right system isn’t just document storage or trial tracking. It’s an intelligent compliance and operations engine that helps your team work faster, smarter, and with greater sponsor confidence. Because in the end, the real question isn’t “can we afford to invest in purpose-built systems?” It’s “can we afford the hidden costs of free?”
For AMCs and sites, the stakes are too high to gamble on “free” systems that compromise compliance and efficiency. And here’s another hidden cost of “free” that often gets overlooked: the learning curve. Every new system demands staff time for onboarding and training. When the platform isn’t intuitive or aligned with research workflows, your team is forced to wrestle with clunky interfaces, inconsistent support, and trial-and-error learning. That’s time that could, and should, be spent on enrollment, data integrity, and patient care.
In other words, “free” isn’t frictionless. It slows your team down at the very moment you need to be accelerating. Free comes at a cost. The real question is:
Will you pay it in missed deadlines, wasted staff time, and increased audit risk, all while climbing the steep learning curve of a system that can’t scale? For organizations committed to operational excellence, the answer is clear: don’t risk paying for “free.”
The Hidden Cost of “Free” eClinical Systems