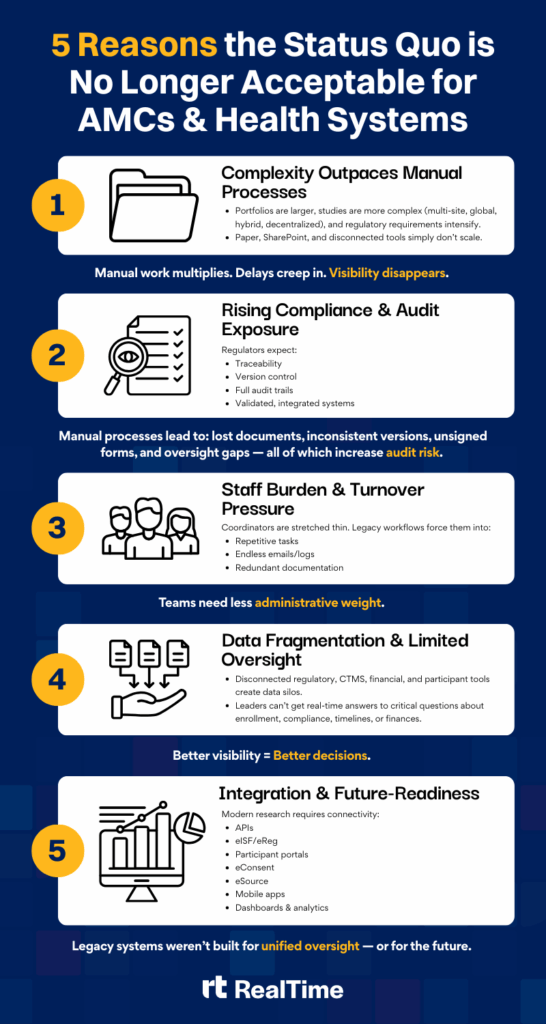
The Challenge: Legacy Systems Everywhere
Why the most advanced AMCs, hospitals, and health systems ow are leaving legacy systems behind.
Academic Medical Centers (AMCs) and health systems are at the forefront of scientific discovery. They run hundreds of trials across departments, manage dozens of sponsor relationships, and balance regulatory, financial, and ethical oversight, often while advancing research that directly improves patient care. But the very systems meant to streamline these operations have become part of the problem. Disconnected technologies, departmental silos, and manual workarounds slow progress, dilute visibility, and strain compliance.
That’s why leading institutions conducting clinical research are adopting a unified Site Operations Management System (SOMS), an integrated, enterprise-grade eClinical command center that connects essential eClinical functions under one roof for total trial oversight.
Within many large AMC and health system research programs, the technologies and workflows supporting clinical trials look something like this:
- Research coordinators are buried in spreadsheets, folders, and paper binders, manually tracking study start-up progress, regulatory documents, participant visits, budgets, and invoices.
- A patchwork of systems. Some content stored in SharePoint libraries, some in local network drives, some “tracked” via manual logs or Outlook tasks.
- Disparate systems that don’t talk to each other. The CTMS may be one platform, the regulatory binder another, the participant portal yet another (or even paper).
- As studies scale (more studies, more sites, more complexity), the lack of integration drives inefficiencies, data errors, delayed timelines, compliance risk and frustrated staff.
For example, the typical point of view:
“We’re still using SharePoint + network drives + tracking spreadsheets. Each new study requires us to manually create folders, copy documents, send email reminders, chase signatures, verify versions, merge data…”
Or:
“Paper regulatory binders, signed consent forms, scanned copies, multiple versions, all of which are hard to search, audit, and we worry about inspection readiness.”
These pain-points are especially acute in complex research environments where trial operations may span multiple departments, investigators, and sites.
The Cost of Fragmentation
Across the industry, research teams manage an average of 22 different systems per study, each with unique logins, workflows, and data silos. For AMCs and health systems, that complexity compounds across departments and therapeutic areas, multiplying inefficiencies and risk. Fragmentation doesn’t just slow research. It undermines it.
- Staff waste hours reconciling data across portals and spreadsheets.
- Investigators lack real-time insight into study performance or patient safety.
- Compliance teams face audit uncertainty due to inconsistent documentation practices.
The result is preventable delay, administrative fatigue, and millions in potential revenue left unrealized.
Oversight Without Overload
Fully validated and 21 CFR Part 11-compliant, SOMS connects your workflows, like source data capture, regulatory documentation, scheduling, and participant payments, and analytics for complete trial management. For AMCs and health systems, this means:
- Real-time PI oversight across all studies
- Centralized, audit-ready documentation
- Automated change logs and eSignatures
- Fewer redundant checks and manual reconciliations
- Fewer redundant checks and manual reconciliations, because SOMS works in concert with Devana’s metrics engine at the back-end
And this is where the real acceleration happens: Layer RealTime-Devana on top of that, and the system shifts from operational to strategic.
Behind the scenes, Devana captures start-up times, enrollment performance, contract turnaround and other key KPIs. By connecting SOMS workflows to the intelligence and benchmarking power of Devana, the result is a system that doesn’t just manage trials; it optimizes them.
Financial and Operational Visibility
Managing a large research enterprise requires precision. Yet disconnected systems make it nearly impossible to measure true cost, revenue, or productivity across programs.
With SOMS, clinical trials leaders gain a unified eClinical command center, connecting financials, scheduling, patient engagement, and performance analytics into a single system. That means leadership finally has a complete, real-time view of:
- Trial budgets and payment reconciliation, from planned spend to actual milestone payments.
- Staff workload and task management, enabling balanced resource allocation, capacity planning, and avoiding over- or under-utilization.
- Enrollment velocity and screening performance, so you can identify bottlenecks, optimize openings, and meet sponsor timelines.
But the power doesn’t stop at visibility. When this operational clarity is paired with the deep intelligence of RealTime-Devana’s analytics engine, capturing historical start-up times, site performance benchmarks, productivity metrics, and network comparators, the result is smart decision-making at scale. With this transparency and insight, you can:
- Allocate resources where they will have the greatest impact
- Forecast more accurately for upcoming studies
- Reduce trial delays
Together, RealTime-CTMS + Devana delivers a complete financial and operational command center that moves from retrospective reporting to predictive, insight-driven decision-making.
Reducing Technology Burden and Burnout
The human impact of system sprawl is significant. Coordinators and PIs spend valuable hours logging into multiple portals, duplicating entries, and reconciling conflicting data. This administrative weight contributes directly to burnout and turnover among research professionals.
By giving staff one login, one system, and one source of truth, SOMS restores focus to what matters most—patients and science.
- PIs regain visibility into study and safety performance.
- Compliance and IT teams regain confidence in validation and security controls.
Unified systems don’t just make operations efficient. They make the people behind them more effective.
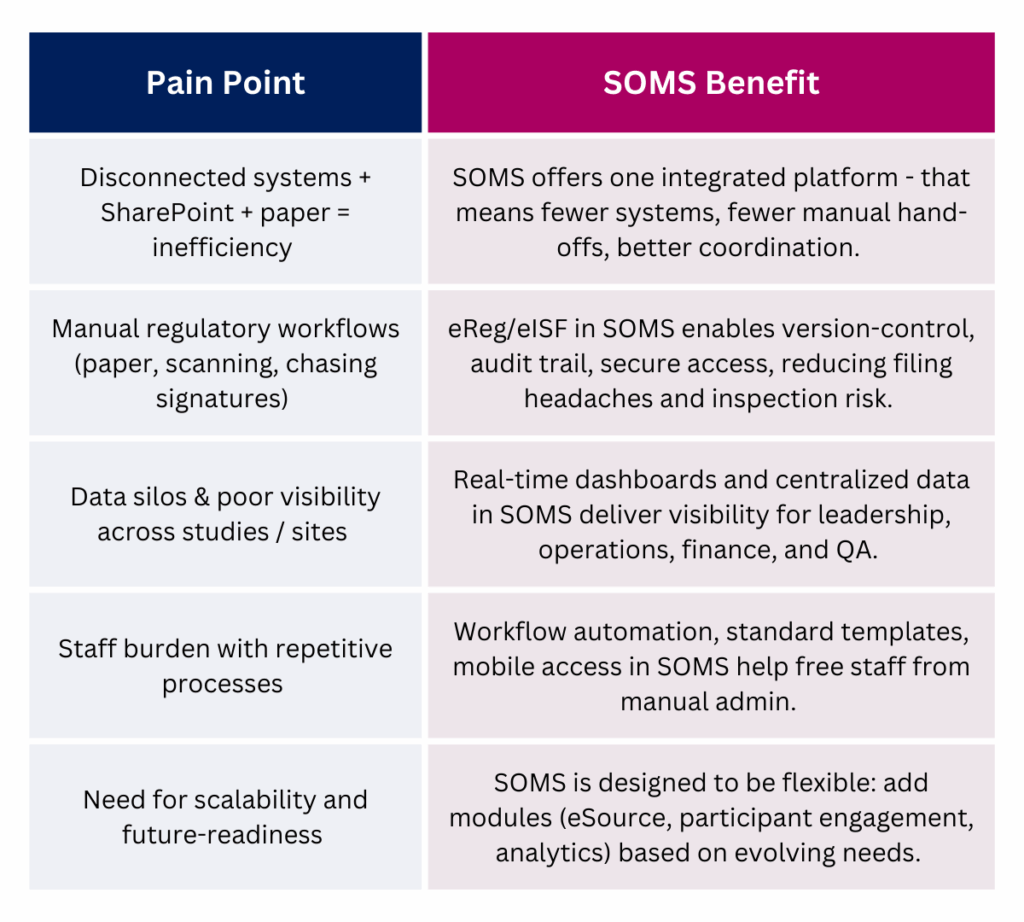
Enter SOMS: A Unified, eClinical Command Center
A Site Operations Management System (SOMS) addresses these challenges head-on.
Rather than piecing together separate systems for CTMS, eReg, eSource, participant communication, payments, scheduling, and analytics, SOMS consolidates industry-leading tools into one essential, interconnected platform.
It functions as a centralized command center for tracking, managing, and optimizing the entire study lifecycle. With real-time data, workflow automation, and streamlined communication built in, SOMS replaces disconnected systems with a single, unified framework.
Key benefits for AMCs and health systems include:
- A fully-featured Clinical Trial Management System (CTMS) module as a foundation.
- eReg/eISF document management: secure, centralized, version-controlled.
- eSource data capture: shifting from paper to electronic at the point of care.
- Participant engagement tools: Participant Portal + eConsent and mobile access.
- Real-time reporting and analytics: dashboards, pipeline visibility, performance metrics.
- One system, fewer logins, fewer silos.
Why AMCs & Health Systems are Switching to SOMS
Real-life Impact
Institutions using SOMS benefit from:
- 5x Faster inspection readiness
- 4x Productivity boost
- 40% Increased revenue
- 20% Increase in rate of study start-up
Tips for Getting Started with SOMS
For AMCs, health systems, and site networks considering the transition to a Site Operations Management System, success starts with clarity, planning, and the right support. Here are practical pointers inspired by insights from RealTime’s Ask the Expert: Tips for Onboarding SOMS guide:
- Inventory your existing state: Map where paper, SharePoint, spreadsheets, legacy CTMS live. Understand workflows and hand-offs.
- Define expected outcomes: What do you want? Better compliance visibility? Faster study start-up? Higher participant recruitment? Single source of truth?
- Change management counts: Staff training, process redesign, and governance will matter. A system alone won’t fix broken processes.
- Measure early and often: Set KPIs for time-to-start-up, document turnaround, visit compliance, recruitment, audits.
- Communicate the story: Use the switch away from paper/SharePoint/legacy systems to communicate to leadership that this is about quality, reputation, and growth.
A Proven, Award-Winning Infrastructure for Research Excellence
RealTime’s SOMS powers 85,000+ studies, supports 7 million patients, and facilitates 350,000 patient visits annually, with over $52 million in participant stipends processed each year.
Recognized with the Clinical Trials Excellence Award, powered by GlobalData, for Innovation in Digital Platforms 2025 and trusted by three of the top five global CROs, nine of the top 10 site networks, and three of the world’s top-ranked hospitals, SOMS delivers unmatched reliability and performance.

Final Thoughts
For AMCs and health systems with growing research portfolios, legacy systems (paper binders, SharePoint libraries, isolated spreadsheets) are simply ill-equipped for the demands of today’s clinical-trial environment. The time, risk, staff burden, and operational inefficiencies are too large.
A unified Site Operations Management System (SOMS) scales seamlessly with complexity, maintaining consistency across departments, geographies, and therapeutic areas. Whether managing 50 studies or 500, administrators gain configurable permissions, standardized data, and centralized oversight.
The smarter play is a centralized, unified system.
By adopting SOMS, organizations unlock a unified platform that centralizes operations, simplifies workflows, provides real-time oversight, and are better positioned for future trials (multi-site, hybrid, decentralized). If your organization is still juggling disparate systems and paper processes, SOMS gives AMCs and health systems the infrastructure for total trial transparency.
Your eClinical Command Center. One Hub. Every Trial. Total Oversight.