Do you have the right site technology to start studies faster, reduce FDA audit risk and lower protocol deviations? Increasingly, clinical research is transitioning from paper-based processes to the adoption of electronic workflows. In fact, since the FDA’s 2003 draft guidance on electronic technology in clinical investigations, the industry has witnessed a surge in the adoption of innovative eClinical solutions. This shift has prompted a reevaluation of traditional practices within the industry. More and more sites are now leveraging eClinical solutions ranging from Clinical Trial Management Systems (CTMS) to eRegulatory and Electronic Source (eSource), to improve patient engagement and overall site operations management.
Amid this transformation, research sites are finding significant benefits in upgrading from paper to digital workflows. Sponsors are also recognizing the value of supporting site-based technology, including expenses related to specific studies. This supports sites in implementing digital solutions that include a range of features to reduce protocol deviations and improve patient recruitment and retention.
In this blog, Clinical Trial Site Management: A Complete Checklist for Clinical Research Sites, we’ll explain the essential eClinical toolkit to help clinical research sites scale faster.
Clinical Trial Management System (CTMS)
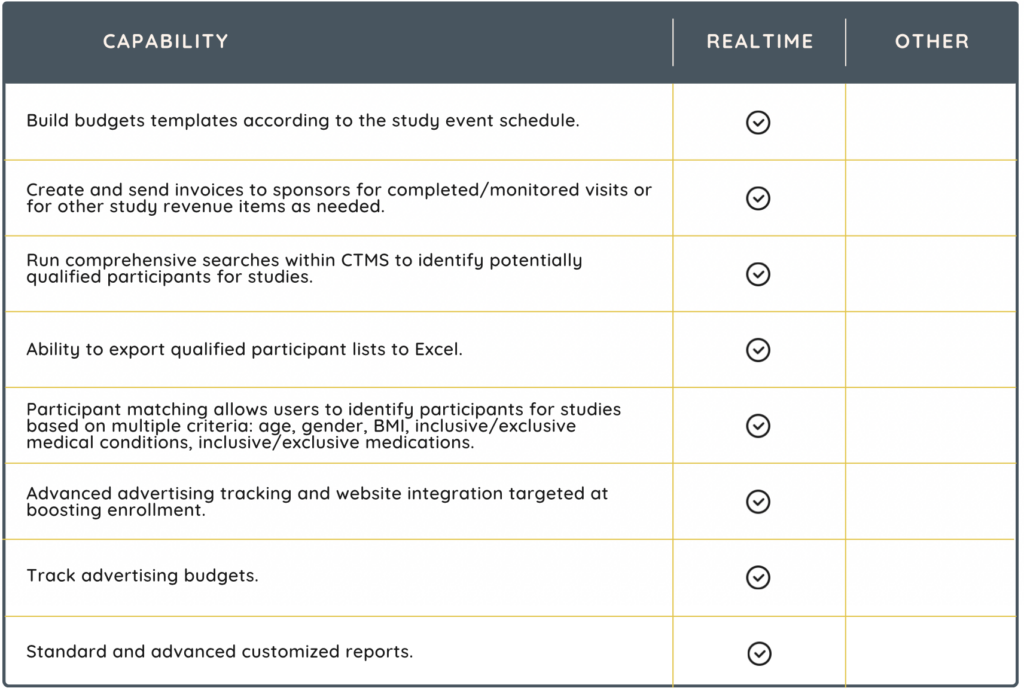
A CTMS is designed to organize and centralize the management of clinical trials for clinical research sites. It serves as a comprehensive tool for planning, tracking, and managing all aspects of a trial, from the initial planning stages to the closeout phase. Sites can simplify study management with a CTMS solution with features that include:
- participant recruitment and scheduling
- site management
- finances
- advanced reporting capabilities
Choosing the right CTMS the first time demands a thorough assessment. An ill-suited CTMS can lead to substantial immediate costs and have enduring negative impacts on your organization. Today’s CTMS extends far beyond standard study management functionalities. These advanced systems equip clinical research sites and staff with comprehensive electronic tools for managing regulatory files, data collection, and communication. Additionally, they integrate financial management capabilities, offering a more encompassing approach to clinical trial oversight.
eSource (Electronic Source)
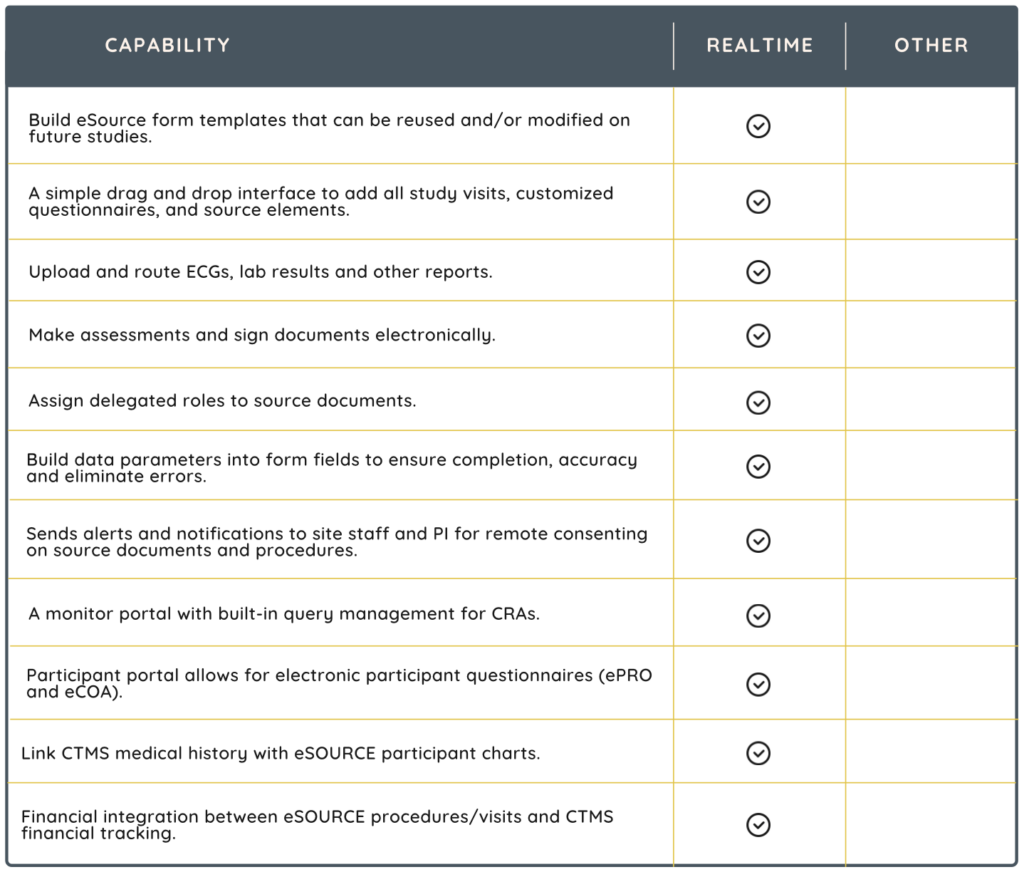
eSource in clinical trials refers to the use of electronic systems to collect, manage, and maintain data generated during the conduct of a clinical trial. In traditional clinical trials, data are often recorded on paper source documents. These are then transcribed into electronic systems for analysis and reporting. eSource eliminates the need for paper by directly capturing data electronically from the point of origin. Unquestionably, eSource improves PI oversight and standardizes source documents across studies, promoting compliance and reducing protocol deviations.
Consider an eSource solution that is scalable to adapt to various trial sizes and complexities. It should also be flexible enough to accommodate different types of clinical research.
eDOCS (eRegulatory)
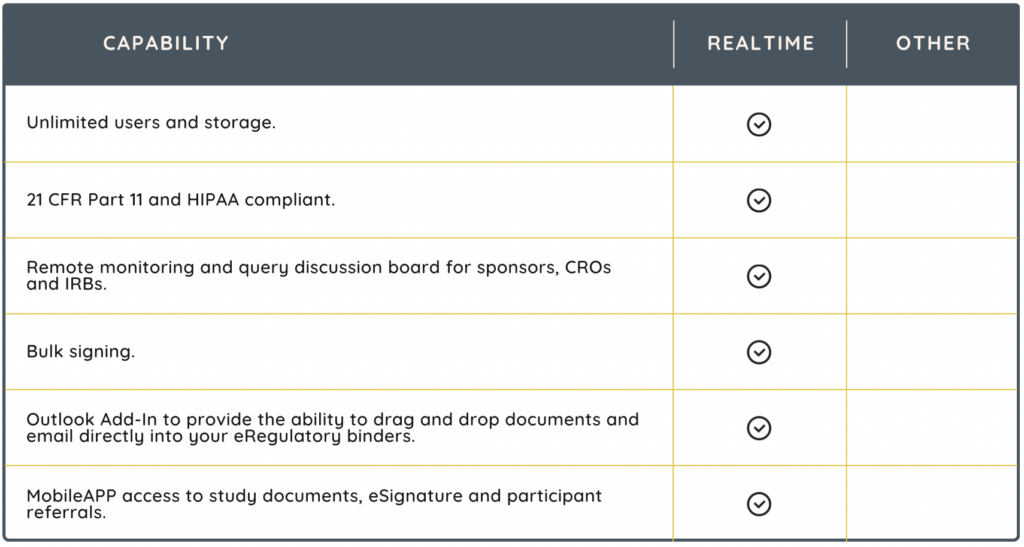
eRegulatory systems provide clinical research sites with a multitude of benefits, key among them being enhanced regulatory compliance and improved operational efficiency. By digitizing and centralizing document management, these systems reduce administrative burden and safeguard data integrity. They also facilitate remote monitoring and accessibility, leading to faster study start-up times and improved collaboration between research sites, sponsors, and CROs.
An eRegulatory solution, like RealTime-eDOCS, can:
- significantly reduce the administrative burden associated with managing regulatory documentation
- ensure compliance to regulatory standards throughout the trial lifecycle
- enhance data quality
- improve collaboration
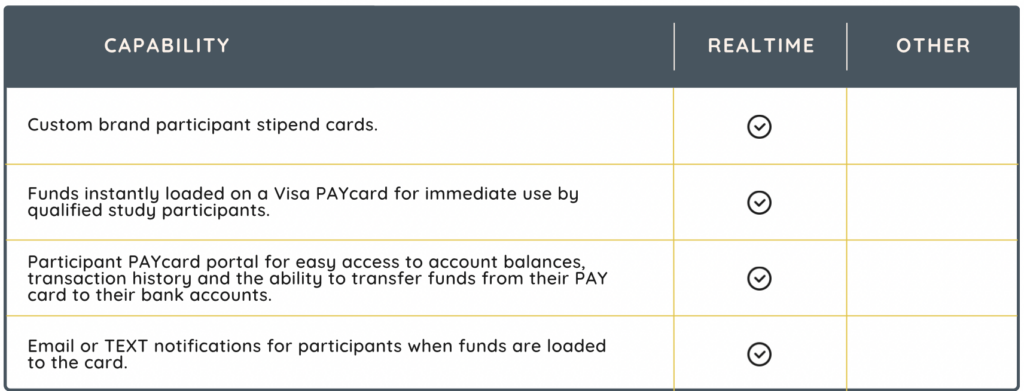
Stipend Solutions
Compensating clinical trial participants for their involvement is a widely accepted and standard practice in clinical research. Stipends compensate participants for their time, and potential discomfort, thereby encouraging more people to enroll and remain committed to a study. Moreover, this compensation ensures that participants are not financially burdened, enabling a more diverse range of participants, crucial for the of the study findings. In addition to meeting regulatory and sponsor requirements, especially in more complex trials, a well-managed stipend system can significantly streamline administrative processes, thereby reducing the burden on research staff.
Stipend solutions, such as RealTime-SitePAY, increase participant compliance and retention by offering fast subject compensation after their visits have been completed.
TEXT
Participant communications can improve retention with appointment reminders and notifications. TEXT communications can be used to:
- reduce no-show rates during a trial
- stay connected with participants in-between visits
- nurture an open pipeline of subject participants after they have completed the study
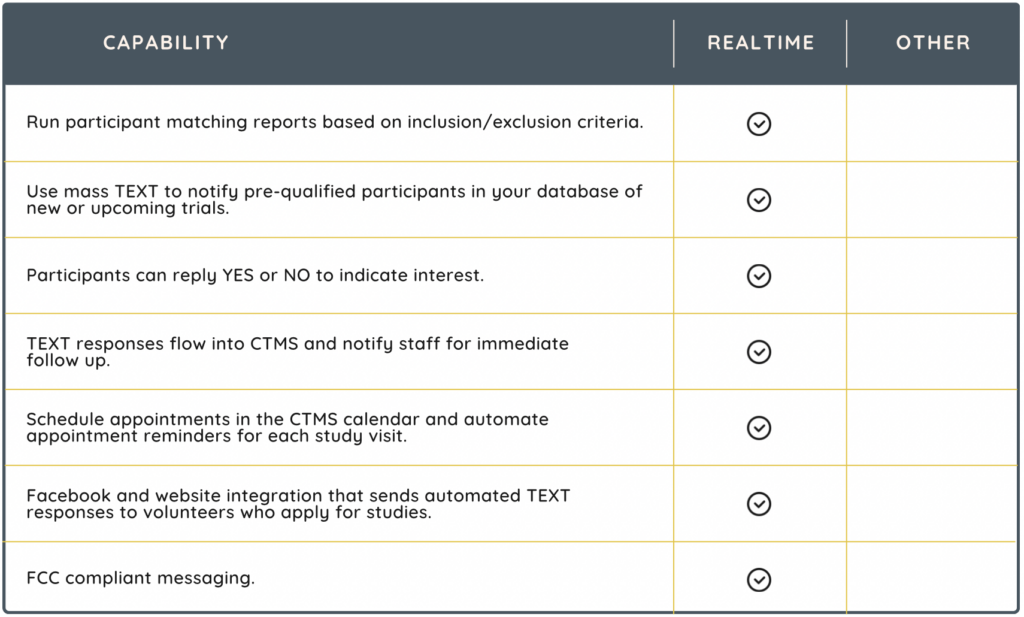
ENGAGE! (Participant portal + eConsent)
Given the significant challenge of patient retention, with a dropout rate considered as high as 30%, having the right site technology becomes crucial for participant recruitment and engagement. Consider a vendor that supports an integrated participant portal and eConsent solution, like RealTime-ENGAGE!
RealTime’s ‘ENGAGE!’ product suite includes MyStudyManager™, the first site-based participant portal that can be used across all clinical research studies to engage with participants in remote settings. ENGAGE! supports decentralized trials and increases the diversity of trial participants. Additionally, for an enhanced experience, the portal integrates eCONSENT, to allow for remote consenting.
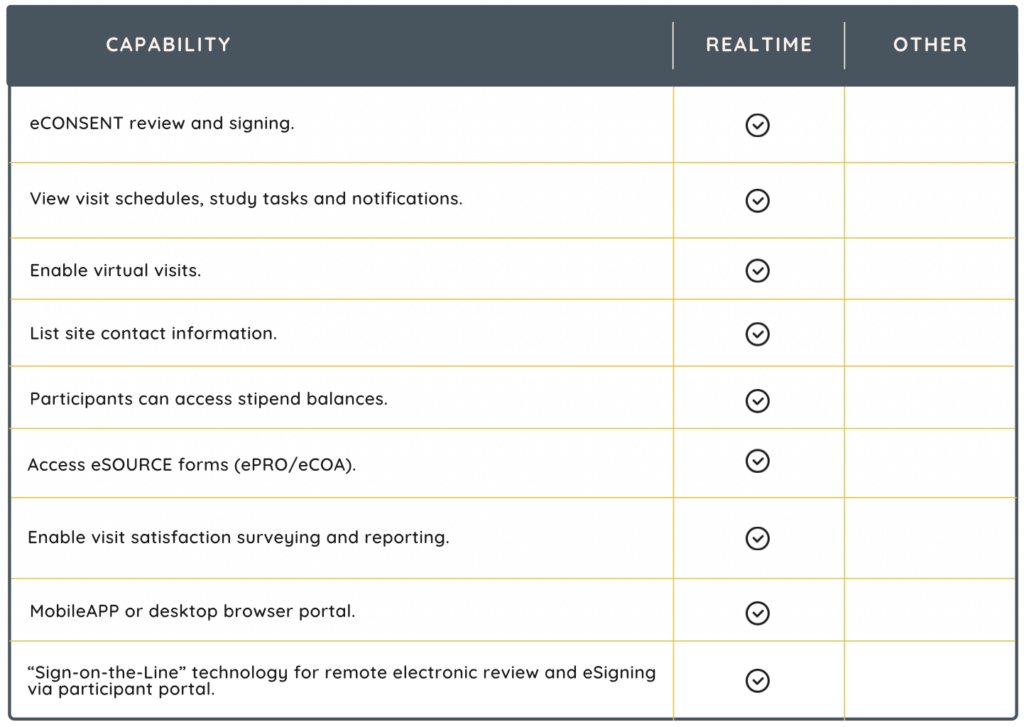
Integrations
Integrations with the following systems make your CTMS even more powerful.
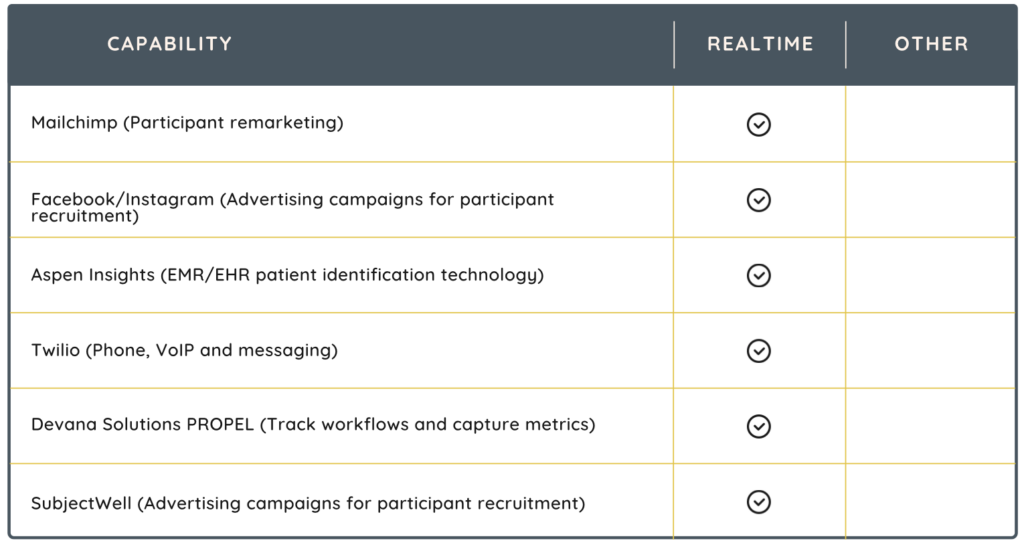
MobileAPP
Imagine managing your entire Site Operations Management System from the palm of your hand. RealTime Software Solutions is the only provider with a MobileAPP for on-the-go management and oversight. With a tablet or phone, you can access studies, participants, calendars, contacts, source documents, regulatory documents and much more.
RealTime’s Mobile APP enhances clinical trial management through various innovative features. Significantly, rather than logging in with password credentials, setting up face or fingerprint recognition can make remembering passwords a thing of the past while also streamlining the eSignature process for documents. Additional features include:
- fingerprint-based document signing
- contact management
- easy navigation of eRegulatory files
- patient referral to studies
- efficient management of signature requests
- compliance with Part 11 validation for eDOCS mobile
- mobile eSource data collection
- access to study documents
- secure password management
Next Steps
RealTime’s Site Operations Management System (SOMS), developed by a former site owner, emerges as a comprehensive eClinical bundle solution and essential eClinical toolkit. SOMS is the first complete Site Operations Management System, designed to centralize and optimize various operational aspects for clinical research sites. The SOMS product suite includes CTMS, ENGAGE!, eSOURCE, eDOCS, SitePAY and TEXT. This fully integrated platform supports clinical research sites in managing all aspects of their operations through a bundled solution. Users can also customize product bundles to align with their unique needs. RealTime products seamlessly integrate as a single system, eliminating error-prone paper processes. Moreover, SOMS is the only solution that offers the convenience of data access through a secure mobile app.
RealTime-SOMS is an all-encompassing solution that facilitates the management of end-to-end clinical trial operations. And with the added advantage of technology costs being reimbursable by sponsors, clinical research sites can have a single provider for the best-in-class eClinical system, built for sites.
Are you ready to upgrade from paper or consolidate your eClinical technology under a single vendor? Explore how RealTime-SOMS can reduce staff burden and help you scale faster.
Read More: Learn how Revival Research Institute experienced 500% revenue growth using RealTime-SOMS, an integrated eClinical bundle solution.
Explore more: Interested in expediting and streamlining study startup, pipeline management and business intelligence? Discover PROPEL, built by Devana Solutions.
Frequently Asked Questions: Clinical Trial Site Management
1. What is clinical trial site management?
Clinical trial site management refers to the coordination of all activities, personnel, technology, and compliance tasks required to effectively conduct a clinical trial at a research site. It includes operations, regulatory, patient engagement, and financial workflows.
2. Why is clinical site management critical to trial success?
Site operations directly impact study timelines, data quality, and patient safety. Well-managed sites launch faster, retain staff longer, enroll more participants, and maintain higher sponsor satisfaction.
3. What should a clinical trial site management checklist include?
A high-quality site management checklist should cover:
Site selection and feasibility
Regulatory compliance (GCP, 21 CFR Part 11)
Training and staff delegation
Participant recruitment and retention plans
Budget and contract review
eClinical systems (CTMS, eSource, eReg)
Quality assurance and audit readiness
4. How do sites prepare for study start-up?
Effective start-up includes:
IRB approval coordination
PI onboarding and delegation of authority
Staff training and protocol review
Site initiation visit (SIV) scheduling
Document collection via eRegulatory or ISF
Using an eClinical platform like RealTime accelerates and automates many of these tasks.
5. What are the biggest risks during site management?
Missed startup milestones
Poor patient recruitment
Regulatory non-compliance
Incomplete documentation
High staff turnover
Mitigating these risks requires proactive planning, clear SOPs, and modern digital tools.
6. How does a CTMS support clinical site management?
A CTMS centralizes study workflows, logs participant visits, handles financial tracking, and provides real-time analytics, making it easier for staff to manage studies efficiently and stay compliant.
7. What is eSource and why is it important at the site level?
eSource refers to the direct digital capture of clinical trial data at the point of care. It eliminates redundant transcription into EDC systems, improves data accuracy, enhances protocol adherence, and allows for faster, remote monitoring. It’s a foundational tool for modern, paperless site operations.
8. What role does eRegulatory (eISF) play in clinical trial site management?
eRegulatory (or eISF) systems digitize essential study documents, track versions, enable electronic signatures, and provide audit trails. They reduce administrative burden, strengthen compliance, and make remote access and inspections easier—particularly across multi-site or AMC-based trials.
9. How can clinical sites improve participant engagement and retention?
Sites can improve participant experience through digital tools like:
eConsent for better understanding and streamlined onboarding
SMS reminders for visits and tasks
Participant portals for document access
Stipend management via tools like SitePay for timely reimbursement
These reduce dropout rates and improve study outcomes.
10. What systems should be integrated for optimal site management?
An integrated eClinical suite should include:
CTMS for site operations and finance
eSource for data capture
eRegulatory for document and compliance management
Engagement tools like eConsent, portals, and messaging
Payments for participant stipends
Mobile access for real-time collaboration
Platforms like RealTime-SOMS, a complete Site Operations Management System, unify these into a seamless environment, minimizing system fatigue and improving productivity across the entire trial lifecycle.


