eRegulatory, often abbreviated as eReg or eReg/eISF (electronic investigator site file), refers to the use of electronic systems to manage regulatory documentation and compliance processes in clinical trials. eReg is an eClinical solution that streamlines the management of critical clinical trial documents. This safeguards that these important documents are securely stored, tracked, and accessed. Today, eRegulatory plays a pivotal role in successful clinical trials. eRegulatory is purpose-built to deliver increased reliability of regulatory workflows, reduce the administrative burden on clinical research teams, and strengthen compliance with regulatory requirements.
In this guide, we’ll explain the evolution of regulatory documentation, key components of eRegulatory, its benefits, as well as key trends driving adoption across the industry. Understanding these elements will provide a comprehensive view of why eRegulatory is considered essential technology for clinical research Academic Medical Centers (AMCs), sites, sponsors, and Contract Research Organizations (CROs) in clinical trials.
Evolution of Regulatory Documentation
The Principal Investigator’s Role in Clinical Trials
Principal Investigators (PIs) are responsible for complete oversight of a clinical trial. They are fully accountable for adhering to the approved protocol, including following the study design, methodologies, and any necessary amendments. Additionally, the PI’s oversight also extends to compliance with regulatory requirements. This involves adherence to Good Clinical Practice (GCP) guidelines, institutional policies, and local and international regulations. This includes compliance with the FDA’s 21 CFR Part 11, which governs electronic records and signatures in the United States, as well as meeting the rigorous standards set by the European Medicines Agency (EMA) and the EU’s General Data Protection Regulation (GDPR).
The PIs role further extends to protecting the rights and safety of trial participants, ensuring that informed consent is obtained, and ethical issues are addressed. PIs must also safeguard data integrity, monitor participant safety by reporting adverse events, and oversee the clinical trial team to guarantee that everyone is adequately trained and compliant with the study protocol.
Finally, PIs must submit timely reports on the trial’s progress, including interim and final reports to sponsors and regulatory authorities, to ensure transparency and compliance. Thus, PIs must maintain accurate records and prepare for regulatory inspections.
Traditionally, regulatory documentation in clinical trials was managed using paper-based systems. These systems involve physical binders and documents stored in filing cabinets, which require significant manual effort to maintain and organize. Manual regulatory processes also present several challenges, including the risk of lost or misplaced documents, difficulties in maintaining version control, and the inefficiency of physical document retrieval and sharing.
These challenges often lead to delays and increase the risk of non-compliance during audits and inspections. The emergence of electronic regulatory solutions marks a significant shift in how regulatory documentation is managed in clinical trials.
What is eRegulatory and eISF?
eRegulatory and eISF (Electronic Investigator Site File) are electronic solutions designed to streamline the management and oversight of clinical trials by automating and centralizing regulatory documentation. These purpose-built solutions offer automated workflows, digital signatures, audit trails, and secure electronic storage, addressing many of the inefficiencies and risks associated with paper-based processes for clinical research sites, sponsors, and CROs.
Understanding the eRegulatory Framework
eRegulatory serves as a comprehensive platform with many integration capabilities that manages all regulatory documents required in clinical trials. The system ensures compliance with regulatory standards and guidelines, such as FDA 21 CFR Part 11 and ICH GCP, through features like electronic signatures, audit trails, and version control. By centralizing document management, eReg reduces the time and effort needed for manual filing and retrieval. It also provides real-time access to up-to-date documents for authorized users, facilitating better collaboration and faster decision-making.
What is eISF in Clinical Trials?
An Electronic Investigator Site File (eISF) is another term for eRegulatory, also known as Site Binder (eBinder) or eTMF. While eReg and eTMF are sometimes used interchangeably, eTMF typically refers to sponsor/CRO solutions managing multi-center studies. Importantly, sites can leverage eRegulatory to manage their own multi-center trials, such as in investigator-initiated studies or when functioning as a coordinating center or academic research organization (ARO). This flexibility supports efficient regulatory management across diverse trial structures.
The eISF is focused on managing site-specific regulatory documents and contains all essential documents that clinical trial investigators and site staff need to manage the trial effectively. These include study protocols, informed consent forms, regulatory approvals, safety reports, financial and legal documents, as well as audit and inspection records. An eISF can also be used to store source or participant documents either for remote monitoring and/or long-term archiving. This system ensures precise documentation management and easy access to essential records, reinforcing the trial’s integrity while maintaining full regulatory compliance.
How eRegulatory Strengthens Clinical Trial Performance
By adopting eRegulatory solutions, clinical research stakeholders can significantly improve operational efficiency across key areas. These systems ensure compliance by maintaining regulatory standards, thereby reducing the risk of penalties and delays. Moreover, manually managing regulatory documents is time-consuming. However, the electronic management of documents automates manual tasks, freeing up staff for more clinical work and accelerating trial timelines. With automated tracking, version control, and audit trails, data integrity is also strengthened for greater reliability and traceability.
While both sponsors and sites utilize eRegulatory systems, their roles and functions within the systems differ. Sponsors focus on oversight, compliance, monitoring, and centralized management across multiple sites, while sites concentrate on document submission, compliance preparation, and day-to-day regulatory tasks. Together, these complementary uses of eReg/eISF ensure a more compliant and coordinated clinical trial process from start to finish.
Next, we will explain how these benefits improve the efficiency and effectiveness of AMCs and clinical research sites, and how sponsors can leverage these advancements to achieve greater success in their trials.
Benefits of eRegulatory for Clinical Research Sites and Academic Medical Centers
For clinical research sites and AMCs, eRegulatory enables sites to create, submit, and manage essential documents electronically, ensuring timely and accurate submissions. eReg also helps sites maintain detailed audit trails, track document versions, and manage electronic signatures. This simplifies the preparation for regulatory audits and inspections. Here are some of the key benefits:
1. Improved Efficiency and Staff Productivity
eRegulatory eliminates the need for paper-based documentation, allowing for quicker and more structured management of regulatory documents. Electronic records can be easily created, updated, and accessed, reducing the time and effort required to handle paperwork. The automation of routine tasks such as document tracking, version control, and approval processes significantly reduces administrative burdens. This enables research staff to focus more on core research activities. For example, many sites that have already adopted RealTime’s eReg/eISF solutions have reported achieving up to a fourfold increase in productivity as a result.

2. Increased Compliance, Data Integrity, and Audit Readiness
eRegulatory platforms are designed to comply with industry standards such as FDA 21 CFR Part 11, ICH GCP, and GDPR. These systems ensure that all regulatory requirements are met, reducing the risk of non-compliance and associated penalties. Comprehensive audit trails further enable detailed tracking of document histories, including who accessed or modified a document and when. This transparency is crucial for maintaining data integrity and facilitating regulatory audits.
3. Centralized Document Management, Accessibility and Security
Tired of repeatedly submitting the same CV, medical license, or training record for different studies? eRegulatory establishes a centralized repository for all regulatory documents, making it easy for authorized personnel to access necessary information from any location. This is particularly beneficial for AMCs with multiple sites or decentralized trial setups. Advanced security features such as encryption, access controls, and secure user authentication protect sensitive data from unauthorized access and breaches. This ensures patient information and trial data are always safeguarded.
4. Streamlined Regulatory Inspections
With all regulatory documents organized and up-to-date, eRegulatory facilitates smooth regulatory inspections in clinical trials. Inspectors can quickly access the required documents, thus reducing the time and stress associated with audit preparations. eReg also allows for real-time updates and notifications, ensuring that all stakeholders are immediately informed of any changes or new requirements. This proactive strategy helps maintain continuous compliance and readiness for inspections.
5. Increased Collaboration and Communication
Because documents can be shared and reviewed in real-time, eRegulatory also supports better collaboration and communication among research teams, sponsors, and regulatory bodies. The ability to access eReg systems remotely, enabling remote monitoring, is particularly valuable in today’s global research environment. It allows team members to collaborate seamlessly regardless of their physical location, promoting a more cohesive, integrated, and patient-centric approach to clinical trial site management.
6. Cost and Physical Space Savings Benefits
By eliminating the need for physical storage and reducing administrative tasks, eRegulatory helps lower operational costs and free up valuable clinic space. Savings can now be redirected towards other critical research activities, as well as the space previously occupied by bulky filing cabinets and paper archives.
Benefits of eRegulatory/eISF for Sponsors and CROs
For sponsors and CROs, eRegulatory is used to manage and oversee all regulatory documents for multiple sites and studies. Importantly, eReg/eISF centralizes documents such as protocols, informed consent forms, and investigator brochures in a single digital repository, ensuring easy access and consistent updates across all sites. The platform also allows sponsors to remotely monitor site activities, review submitted documents and ensure timely completion of regulatory submissions. This helps in maintaining uniform standards across all trial locations. Here are some key benefits of eReg/eISF for sponsors and CROs:
1. Centralized Document Management
With eReg/eISF, sponsors and CROs can centralize all regulatory documentation across multiple sites, ensuring consistency and uniformity in document management. This centralization reduces the risk of discrepancies and ensures that all sites are working with the most current and approved documents.
2. Rapid Start-up and Seamless Site Adoption
eReg/eISF systems streamline the setup and management of clinical trials, offering sponsors the ability to rapidly create and deploy any type of study without the need for extensive study build. Utilizing documentation and automation templates, sponsors can quickly establish Investigator Site Files (ISFs) and effortlessly transition eISF control to any site worldwide. This process is supported by built-in training, validation and comprehensive support, improving site adoption.
3. Active Site and Investigator Engagement
From the initiation of the trial, eReg/eISF empowers sites to effectively qualify, initiate, and support investigators and study teams. This is facilitated through role-based automation for delegation, credentialing, training, and access to the latest protocol versions, all accessible via a mobile clinical portal. Such capabilities ensure that sites can activate and manage investigators efficiently, significantly reducing the time from start–up to study conduct.
4. Enhanced Clinical Trial Management and Oversight
eReg/eISF enables sponsors and CROs to monitor site compliance and performance in real-time. This helps maintain that all sites adhere to regulatory requirements and study protocols. Real-time updates and notifications also allow for the prompt identification and resolution of issues, minimizing delays and ensuring smooth trial progression.
5. Enable Remote Monitoring
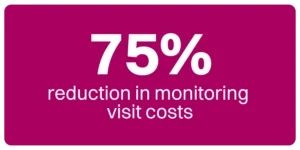
With eReg/eISF, sites can better meet sponsor and CRO expectations and are always prepared for monitoring visits, including remote monitoring. eReg/eISF facilitates seamless sharing of documentation from various sources such as IRBs, vendors, and labs. The system helps maintain impeccable file organization through folders, keywords, and consistent naming conventions. This ensures that all documentation is easily accessible and audit-ready. The platform also supports the accurate completion of regulatory requirements. This is done through workflows designed for tasks like delegating responsibilities, maintaining living logs, capturing eSignatures, and more. These features not only enhance on-site monitoring but also enable effective remote monitoring in clinical trials, allowing sponsors and CROs to review documentation in real-time without the need for physical site visits. For example, sponsors using RealTime’s eReg/eISF report up to 75% reduction in monitoring visit costs using the software.
Trends Driving Adoption of eReg/eISF in Clinical Trials
Regulatory Pressures Driving eReg/eISF Adoption
The adoption of eReg/eISF systems in clinical trials is being driven by several key trends. Increasing regulatory scrutiny and the need for compliance with complex regulatory requirements, such as FDA 21 CFR Part 11 and ICH GCP, are compelling organizations to seek more methodical and reliable ways to manage regulatory documentation. The growing volume and complexity of data in clinical trials necessitate systems that can handle large amounts of information while ensuring data integrity and accessibility.
Restrictions Limiting On-site Visits
Additionally, many sites are increasingly restricting on-site monitor access due to cost, inconvenience, or local site policies. eReg/eISF platforms address these challenges by offering remote access to critical documentation. This safeguards regulatory compliance while accommodating site-specific needs.
Impact of Site Network Consolidation on eReg/eISF Adoption
Moreover, the trend towards site network consolidation is playing a significant role in the shift to eReg/eISF platforms. As smaller sites join larger networks or merge with other entities, there is an increased need for standardized and centralized document management processes across multiple locations. eReg/eISF solutions enable these organizations to maintain consistency, compliance, and oversight, even as they scale and integrate new sites.
Decentralized Trials and the Shift to Electronic Solutions
Additionally, the rise of decentralized and remote clinical trials, accelerated by the COVID-19 pandemic, has highlighted the need for electronic solutions that enable real-time access to documents and seamless collaboration among geographically dispersed teams. The overall drive towards digital transformation in the healthcare and life sciences sectors also supports the move from paper-based to electronic systems. Altogether, electronic solutions in clinical trials, also known as eClinical solutions, improve efficiency, reduce administrative burdens, and strengthen data accuracy and security. These trends reinforce the importance of adopting eReg/eISF systems to meet the evolving demands of modern clinical trial management.
3 Key Takeaways
eReg/eISF systems are increasingly recognized as essential tools in the management of regulatory documentation and compliance processes in clinical trials. Organizations that leverage these technologies can more effectively manage the growing volume of regulatory documentation, meet tight deadlines, and ensure that all trial activities are aligned with regulatory expectations. As the adoption of eRegulatory solutions grows, the impact on clinical research sites, site networks, academic medical centers, sponsors and CROs is increasingly evident through substantial data-driven outcomes. Designed to scale with research operations, eReg/eISF maintains efficiency and compliance across complex or multi-site studies, while also reducing administrative burdens.
1. Enhancing Organizational Reputation Through eRegulatory Solutions
The reputation of an organization in clinical research is closely tied to its ability to conduct trials that meet the highest regulatory standards of quality and compliance. By adopting eReg solutions and partnering with a reputable technology provider, organizations can demonstrate their superior performance in regulatory management. This not only enhances sites standing with sponsors, CROs, and regulatory bodies, but also builds trust with trial participants and the broader community.
2. Tangible Benefits and Data-Driven Outcomes of eRegulatory Adoption
For instance, sites and AMCs that have already adopted RealTime’s eReg/eISF solutions have demonstrated that implementing the platform helped them achieve five times inspection readiness. This increase in inspection readiness directly contributes to, on average, a 20% increase in faster study start-up timelines. These tangible benefits highlight the critical role that eReg/eISF plays in improving clinical trial processes and highlighting a site’s key strengths.
3. Choosing the Right eRegulatory Technology Partner
Selecting the right eReg/eISF technology partner is a critical decision for organizations. It’s important to choose a partner that not only meets current needs but also offers comprehensive training and ongoing compliance support. The right partner will ensure that all users are fully equipped to utilize the system effectively, minimizing errors and enhancing operational efficiency.
Frequently Asked Questions: eRegulatory & eISF in Clinical Trials
1. What is eRegulatory (eReg) in clinical trials?
eRegulatory, or eReg, refers to digital systems used to manage essential regulatory documents in clinical trials. It replaces paper binders with secure electronic filing, automates workflows, and ensures compliance with standards like FDA 21 CFR Part 11 and ICH GCP.
2. What is the difference between eReg, eISF, and eTMF?
eReg/eISF (Electronic Investigator Site File): Used by clinical sites to manage site-level regulatory documents.
eTMF (Trial Master File): Used by sponsors and CROs to manage documentation across all sites in a trial.
Sites may also use eReg to function as coordinating centers or manage multi-site investigator-initiated studies.
3. What are the benefits of using eRegulatory systems at clinical research sites?
Faster startup and fewer delays
Centralized document access
Real-time collaboration across teams
Improved audit readiness
Reduced administrative burden
Enhanced data integrity and compliance
4. How does eISF support Principal Investigators (PIs)?
PIs are responsible for regulatory oversight, compliance, and participant protection. eISF helps maintain accurate, timely documentation, supports remote monitoring, and ensures readiness for audits and inspections.
5. Is eRegulatory software compliant with regulatory standards?
Yes. Systems like RealTime’s eReg/eISF are built to comply with FDA 21 CFR Part 11, ICH GCP, GDPR, and other global regulatory requirements. Features include audit trails, role-based access, electronic signatures, and version control.
6. Can sponsors and CROs use eRegulatory systems?
Absolutely. Sponsors and CROs use eReg to:
Monitor document submissions across sites
Remotely access site files
Track compliance in real time
Standardize document templates
Accelerate site activation timelines
7. How does eRegulatory improve inspection readiness?
eReg enables consistent versioning, audit trails, and automated alerts to keep documentation current. It also allows for remote access during inspections, ensuring nothing is misplaced or out of date.
8. Can eReg/eISF be used in decentralized or multi-site trials?
Yes. eReg systems are particularly valuable in decentralized trials, AMCs, and site networks. They ensure consistency across all sites and allow for real-time monitoring regardless of physical location.
9. What performance impact can sites expect from eReg adoption?
Sites using RealTime’s eReg/eISF have reported:
Up to 4x improvement in staff productivity
5x greater inspection readiness
20% faster study startup timelines
75% reduction in monitoring visit costs
10. What features should I look for in an eRegulatory system?
FDA 21 CFR Part 11 compliance
Built-in audit trails and eSignatures
Secure remote access
Centralized document storage
Role-based automation and delegation
API or system integration support
Sponsor/CRO document sharing capabilities
About RealTime eClinical Solutions
From the pioneers of RealTime-eDocs and Complion eISF, our intuitive eReg/eISF electronic document management and regulatory systems are tailored specifically for AMCs, sites, site networks, and sponsors. 21 CFR Part 11 complaint, our eClinical solutions help eliminate paper clutter. The platforms streamline document storage, retrieval, and collaboration across site teams and AMC departments. As clinical trial requirements become increasingly complex, adopting an eReg/eISF solution is crucial for maintaining competitiveness, ensuring compliance, and boosting your organization’s reputation.
Experience the benefits firsthand. Schedule a demo with RealTime-eClinical Solutions today to see the eReg/eISF platforms in action. Schedule a demo here.

