By design, clinical trials are a long and complicated process involving a seemingly endless number of moving parts. It can be difficult to manage everything effectively, especially with the rise of decentralized study teams and more complex trial designs and protocols. For Academic Medical Centers (AMCs) in particular, project management across the entire clinical trial process often proves to be a time-intensive challenge.
RealTime-Devana’s category-defining technology offers real-time oversight into trial status, custom integrations, and powerful collaboration tools. Serving as the central hub in any given clinical trial tech stack, Devana combines all the key data in one place—providing AMCs with total performance transparency, automated workflows, and reduced workloads overall.
Project Management and Trial Oversight
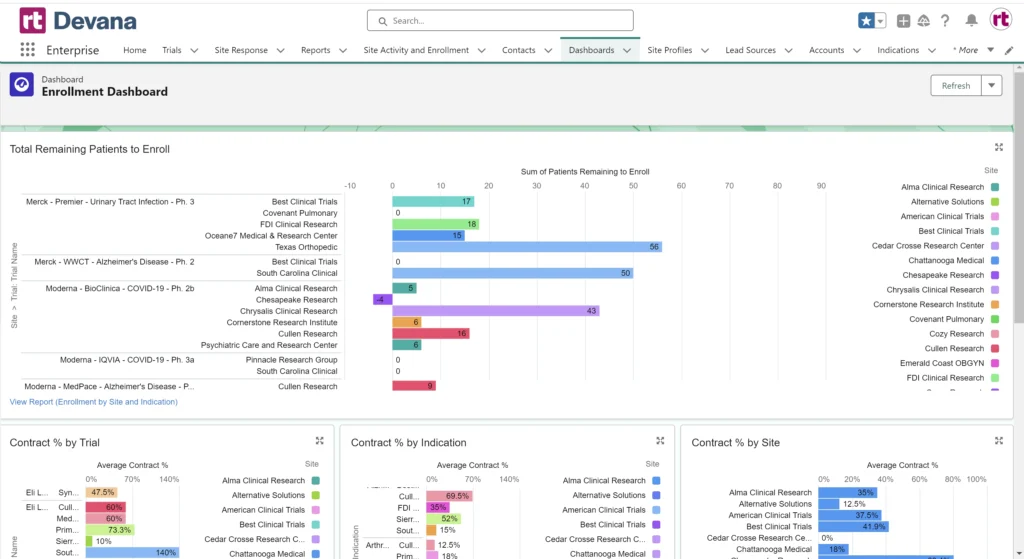
Devana Enrollment Dashboard
Devana is a powerful project management tool that enables teams to establish improved workflows throughout the entire clinical trial process. Promote buy-in and accountability with transparency into trial status, outstanding tasks, enrollment goals, and performance expectations both organization-wide and for each individual study team. View at a glance which trials are proceeding on schedule, and which are not – allowing for timely interventions to get you back on track.
With Devana, AMCs can track enrollment, activity, timing, and performance based on trial, study team, sponsor, and more. Create customized dashboards and reports using real-time data for powerful metrics and improved trial management. With oversight into every aspect of clinical trials in the system, Devana makes it easy for teams to give guidance and resources where needed.
Study teams also gain visibility and accountability into the study startup progress, enrollment performance goals, and overall trial status. Devana allows them to respond to notifications of the latest trial opportunities, along with other task alerts tied to specific trials. Threaded chat conversations streamline communication and allow for real-time collaboration and document sharing. Connect teams no matter their location with Devana.
Seamless Integrations
With the wide array of disjointed technology such as CTMS, IRB, contracts and budgets, grant management, and more, Devana’s open API gives AMCs the power to seamlessly integrate with the tools their team is already using. Integrations with mission-critical systems allow data to flow and synchronize across all platforms, providing powerful oversight for central research teams. Paired with Devana’s custom dashboards and reports, cross-platform data can be easily shared with key stakeholders anytime.
Acting as the hub of the clinical trial tech stack, Devana’s custom integrations bring together all the clinical trial data from IRB systems, contract and budget programs, regulatory systems, and beyond—all in one place. By tying together disparate systems in one centralized and easy-to-use platform, AMCs can eliminate duplicate data entry and boost trial cycle times.