Behind the Software: Becoming a Great Place to Work
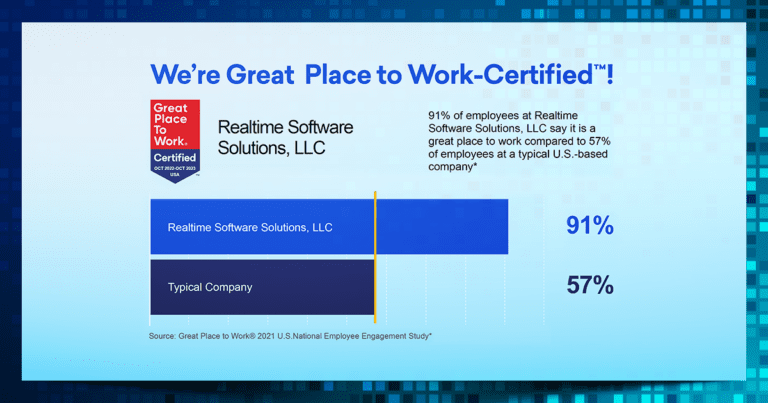
RealTime has always believed that our people are our greatest asset. We are thrilled to announce that our commitment to fostering a positive and inclusive workplace culture has been recognized once again by a Great Place to Work® Certification! In this issue of Behind the Software: Becoming a Great Place to Work, we’re spotlighting the culture