
Bring in the study design from the sponsor’s EDC via CDISC ODM

Quickly match eSource forms to EDC fields using the Form Builder

Send clean, mapped ODM files back automatically
Clinical sites waste countless hours re-entering data from eSource into sponsor-provided EDC platforms. This manual process leads to transcription errors, delayed patient safety data, more queries, and slower study closeouts.
EDC Connect is the only solution that eliminates duplicate data entry by mapping and exporting eSource data directly into any ODM-compatible EDC – within minutes.
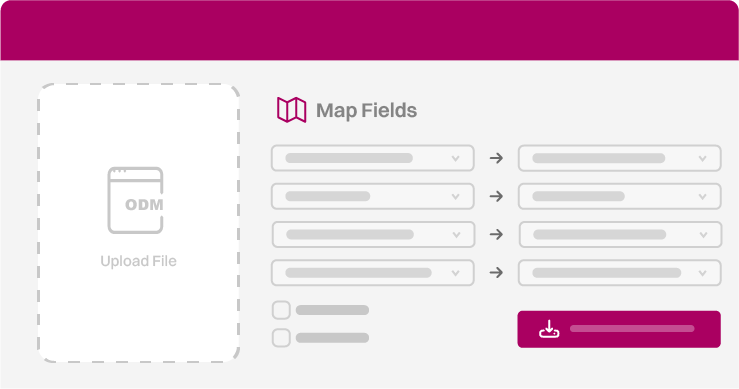
Enter data once in eSource and let EDC Connect handle the rest.
Minimize transcription mistakes.
Shorten timelines and enable faster query resolution.
Fully aligned with 21 CFR Part 11 requirements.
Works with leading EDCs: Medidata Rave, Oracle Inform, and more.
| Cookie | Duration | Description |
|---|---|---|
| cookielawinfo-checbox-analytics | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Analytics". |
| cookielawinfo-checbox-functional | 11 months | The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". |
| cookielawinfo-checbox-others | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Other. |
| cookielawinfo-checkbox-necessary | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookies is used to store the user consent for the cookies in the category "Necessary". |
| cookielawinfo-checkbox-performance | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Performance". |
| viewed_cookie_policy | 11 months | The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. It does not store any personal data. |