
RealTime-Devana’s category-defining software helps standardize and accelerate clinical trial operations, making trials more efficient and reducing cycle times. Stay competitive and transform your organization’s trial performance end-to-end with improved pipeline management, automated study start-up workflows, and real-time metrics capture and analytics.
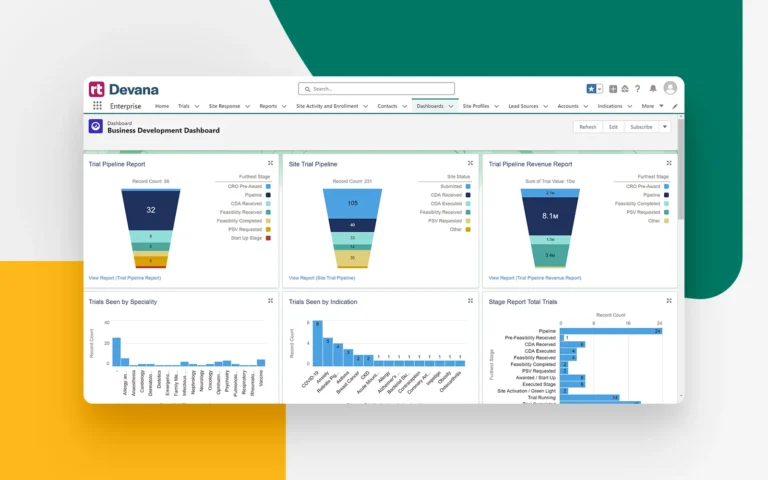
Track and monitor new trial leads from CROs and sponsors to build and develop your study pipeline.
Automatically log business development emails and calls, tying them directly to specific trial opportunities, contacts, and more for improved oversight.
Identify emerging industry trends and create custom reports to help drive buy-in and awareness across your organization.
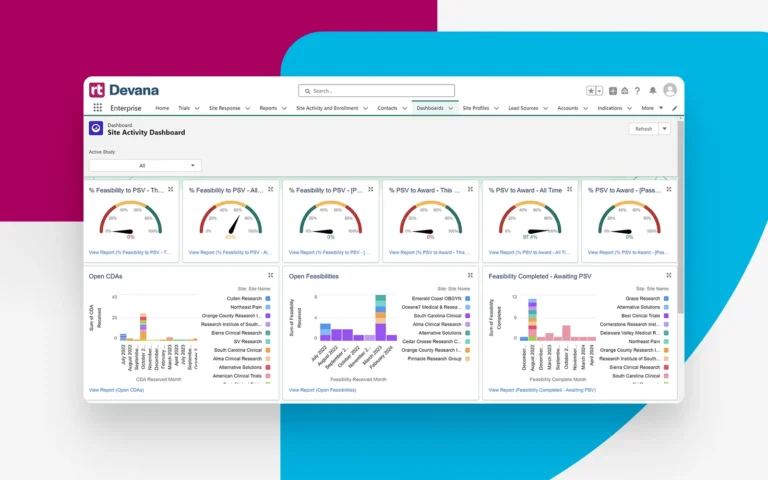
Gain tangible insights from both current and historical site activities and performance.
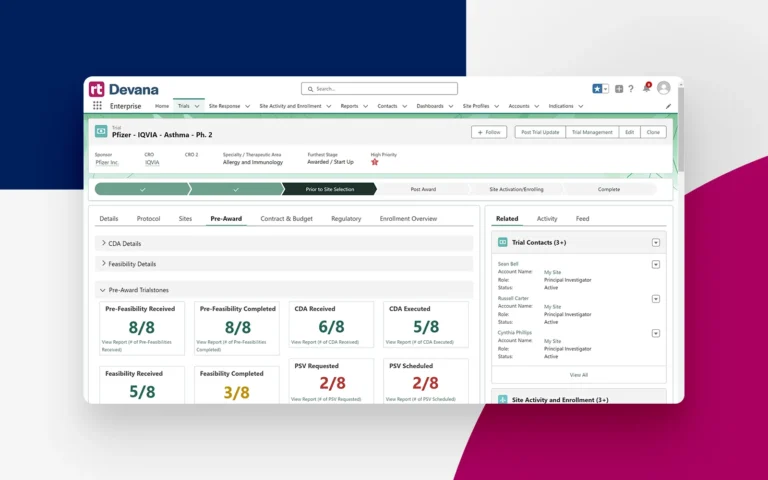
Standardize and streamline study start-up workflows with intelligent automation, eliminating redundant tasks and unnecessary status update emails and meetings.
Easily track trial progress and completion of study start-up milestones from CDA and Award Letter to Site Activation and beyond.
Keep trials on-time with custom alerts and threaded chat conversations, ensuring everyone is in the loop on updates and outstanding tasks.
Improve accountability with transparency into trial status, outstanding tasks, patient enrollment goals, and performance expectations organization-wide and for each site or study team.
Automatically capture timing metrics for all start-up milestones such as contract and budget turnaround between your organization and sponsors and CROs. Create customized views using real-time data and metrics.
Import trial screening and enrollment data from your CTMS to capture overall trial progress and enrollment metrics at an organization-wide scale.
Store all trial-related contacts and documents in one secure place. Associate them with specific records such as a trial or site, automatically track revisions, and easily share updates directly within the platform.
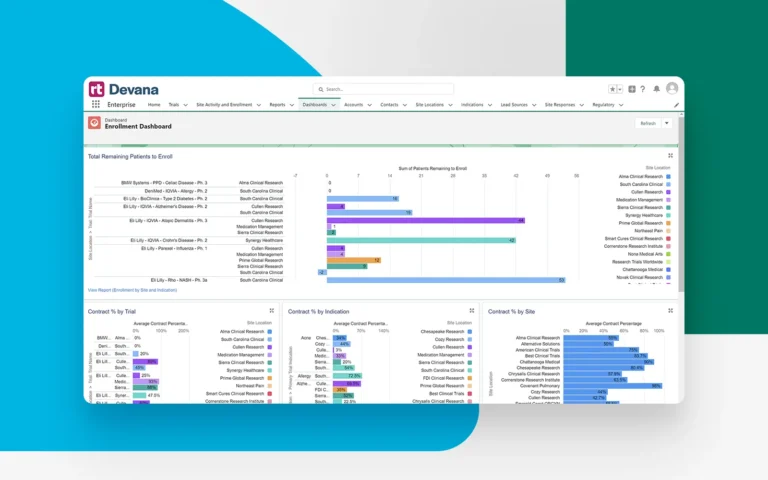
Capture overall performance metrics both within Devana and via integrations with other systems such as CTMS, allowing for full-cycle data display and analysis. In fact, every data point captured within Devana is reportable.
Share and analyze process and performance data in real time across all stakeholders to track trial progress and make more informed decisions based on the latest metrics.
Powerful dashboards and reports make it easy to share insights and historic data with CROs and sponsors, helping win more opportunities based on your organization’s past accomplishments.
Empower your sites to actively help build the trial pipeline, digitally respond to trial opportunities, and communicate in real time with your central research team. Enhance site and organizational operations, allowing data to flow between stakeholders.
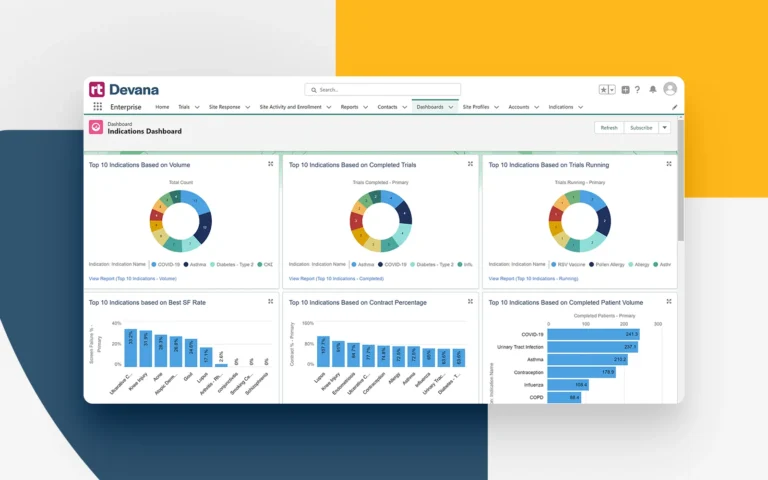
Seamlessly manage and monitor your expansive network of owned or affiliated sites and study teams. Track site interest in trials, study startup activity, patient screening and enrollment progress, and beyond.
Rapidly distribute new trial opportunities directly within the platform, allowing for more accurate PI/site selection based on capabilities, therapeutic expertise, physical site facilities, and more. Automatically collect timing and response data for easy monitoring and reporting.
Harness powerful real-time data analysis and reporting, providing unmatched clarity into processes and performance at the site and network-wide level.
| Cookie | Duration | Description |
|---|---|---|
| cookielawinfo-checbox-analytics | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Analytics". |
| cookielawinfo-checbox-functional | 11 months | The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". |
| cookielawinfo-checbox-others | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Other. |
| cookielawinfo-checkbox-necessary | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookies is used to store the user consent for the cookies in the category "Necessary". |
| cookielawinfo-checkbox-performance | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Performance". |
| viewed_cookie_policy | 11 months | The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. It does not store any personal data. |