- Better Research.
- Better Business.
- Better Outcomes.
Studies
0
Sites
0
Patients
0
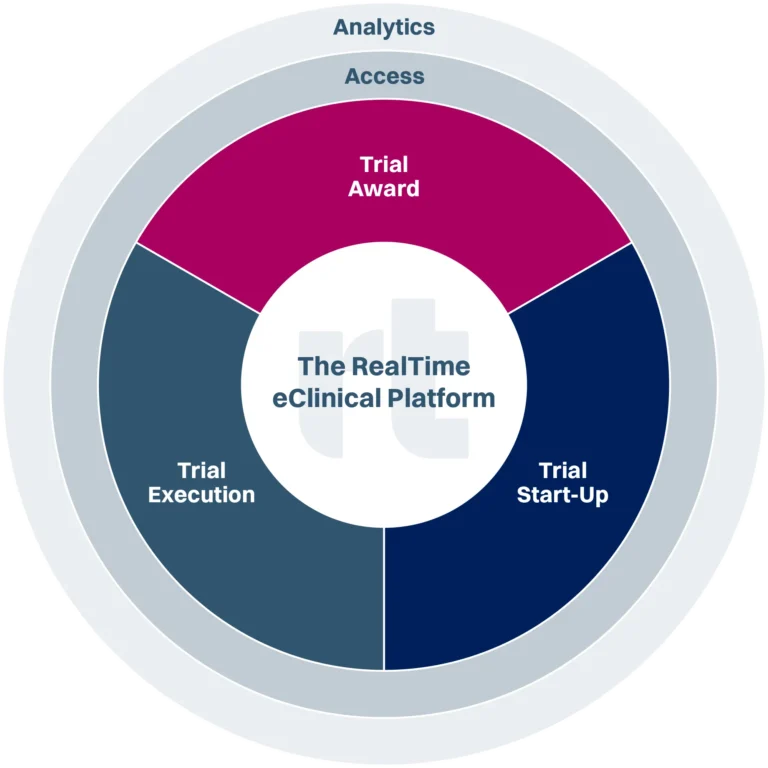
Best in class solutions across the clinical trials journey.
Trial Execution
Access & Analytics
Your success is our success
“
They are open to our multiple product enhancement suggestions, and I value them as a partner.
”
DaveCIO, Large Site Network
“
RealTime staff has been helpful with any question we have. They are knowledgeable and always willing to help work through questions.
”
CindyResearch Manager
“
RealTime-CTMS is a very well-built software. [It] was built by former research coordinators. We have yet to find something we don't like about the software.
”
BradAccounting Manager
“
This software has all the tools that we need to conduct our clinical trials without any stress. I love everything about this software. It helps to make your work flow a lot easier.
”
RobertSite Director
“
Having all of the tools available in one spot makes it easy and efficient to use. RealTime really does it all and does it well. It's a one-stop shop for keeping our studies well organized!
”
AmyCRC Manager
Request a Live Demo
Featured Resources
Institutional review boards (IRBs) are ethics review committees that ensure human research subjects’ rights are protected, as well as the rights of the patients who ultimately benefit from the research. Operating under US federal regulations, state laws, and institutional policies, the IRB has the authority to approve, require modification to, and disapprove research. IRBs are an important part of regulatory efforts and their approval is required for all human research. Here's a quick overview of IRBs and the expectations for working with them.
Tekton Research wanted to enable full remote monitoring and needed to be able to upload, manage, and share source documents with monitors and sponsors without redacting sensitive patient health information.
We explore Coastal Pediatric Research's journey to adopting Complion’s eRegulatory solution which has enabled the site to increase efficiency, greatly improve time management, and eliminate duplication of work while conducting their clinical research studies. In the first of this three-part series, we take a look at the selection phase of the adoption of an eRegulatory solution.













